Current location: Home > NEWS > Industry news
NEWS
PRODUCTS
A novel antibody-drug conjugate targeting HER2 :Trastuzumab -Deruxtecan, has durable anticancer activity!
News source: Release time:[2022-12-13]
In the phase 3 DESTINY-Break04 trial (NCT03734029), the benefits shown by fam trastuzumab deruxtecan-nxki (Enhertu) and the chemotherapy selected by doctors confirmed that HER2 low metastatic breast cancer patients were classified into a separate subset. According to the data of DESTINY-Break04, in August 2022 [1].
Data of DESTINY-Break04
These patients do not respond well to standard-of-care chemotherapy. Having another drug, such as Trastuzumab - Deruxtecan, is very important for this patient group. Katherine Tkaczuk, M.D., Said in the interview from OncLive after the breast cancer webinar of cancer institutions.
In the interview, Tkaczuk discussed practice-changing data across breast cancer subtypes at the 2022 ASCO Annual Meeting, including data from the DESTINY-Breast04 and Phase 3 PALOMA-2 trials (NCT01740427), and CDK4/6 using of Inhibitors and PARP inhibitors in certain breast cancer patients. Tkaczuk is director of the Breast Evaluation and Treatment Program at the Marlene and Stewart Greenebaum Cancer Center at the University of Maryland School of Medicine. (excerpt)
Q:How can subcutaneous Pertuzumab (Perjeta) and Trastuzumab (Herceptin) help improve quality of life in patients with HER2-positive breast cancer?
Tkaczuk: I'm happy to discuss the data of the [phase 3] FeDeriCa trial [NCT03493854] and Phesgo [subcutaneous Pertuzumab, Trastuzumab and hyaluronidase-zzxf] . This is a very interesting study comparing the pharmacological equivalence of subcutaneous formulations for Trastuzumab and Pertuzumab to intravenous [IV] formulations. Phesgo is now FDA-approved for the treatment of [HER2-positive] breast cancer in the adjuvant, neoadjuvant and metastatic settings.
In terms of [Phesgo's] usage, it's still underused. The FeDeriCa test showed that this subcutaneous formulation was equivalent [to the IV formulation]. Most patients in the FeDeriCa and [Phase 2] PhranceSCa trial [NCT03674112] preferred the subcutaneous formulation. This is easier for the patient. They did not have to stay in the infusion center to receive intravenous Trastuzumab and Pertuzumab. With regard to the quality of life of the patient, this is something to consider. Many patients are being switched to subcutaneous formulations of Trastuzumab and Pertuzumab.
Also, I discuss the [Phase 3] KATHERINE trial [NCT01772472] in HER2-positive breast cancer patients with residual disease after neoadjuvant chemotherapy. In this trial, the antibody-drug conjugate [ADC] Trastuzumab emtansine [T-DM1; Kadcyla] was compared with Trastuzumab in adjuvant therapy. These figures are not new, although there have been some additional updates. Currently, for patients with residual disease after neoadjuvant chemotherapy, which is indeed the only approach, patients should be advised to receive adjuvant T-DM1 therapy. The trial showed an improvement in event-free survival [EFS] in patients treated with ADC compared to Trastuzumab.
Q: What stands out from the efficacy data observed with Trastuzumab-Deruxtecan in the phase 3 DESTINY-Breast03 trial in HER2-positive breast cancer?
Tkaczuk:DESTINY-Breast03 is a randomized trial comparing Trastuzumab-Deruxtecan with T-DM1 in patients with HER2-positive advanced breast cancer. These data have been published and there was a significant improvement in [progression-free survival (PFS)] with Trastuzumab-Deruxtecan. This treatment also significantly increased response rates compared with the control group. This is in HER2 overexpressing or positive breast cancer, as we often refer to these patients, HER2 expression in these patients must be 3+ by immunohistochemistry [IHC] or positive by fluorescence in situ hybridization.
Q: Marlene of the University of Maryland and Ghazal Kango, MD of Stewart Greenebaum Cancer Center, further elaborated the clinical significance of HER2 low. What does DESTINY-Break04 mean by identifying HER2 low breast cancer patients as a separate subset of patients?
Tkaczuk: DESTINY-Break04 test is changing the practice. The previous knowledge about HER2 positive breast cancer can be traced back to the initial key test conducted in a metastatic environment using Trastuzumab, followed by other tests for patients with low HER2 expression, including [Phase 3] B-47/NRG test I participated in [NCT01275677]. This is a negative experiment. This is an auxiliary test for HER2 low breast cancer patients to evaluate whether Trastuzumab is beneficial to HER2 low breast cancer patients.
The subjects included breast cancer with positive or high-risk lymph nodes and negative lymph nodes. Patients were randomly assigned to receive standard treatment chemotherapy with or without trastuzumab for 1 year. The test was negative for invasive disease-free survival and OS. So far, we have no evidence from clinical trials that anti HER2 therapy is beneficial to breast cancer patients with low HER2 expression.
DESTINY-Break04 trial specifically studied patients with metastatic HER2 low breast cancer. In this large phase 3 trial, patients were randomly assigned to receive Trastuzumab -Deruxtecan treatment or chemotherapy of the doctor's choice. In terms of enrolled subjects, the trial allows for previous chemotherapy for metastatic diseases. They also allow patients to receive at least first-line hormone therapy. Including patients with stable brain metastasis, most DESTINY-BREAST tests are the same.
The primary end point was PFS in HR positive patients. The investigators also looked at OS and PFS secondary endpoints for all patients. Usually, these patients received single drug chemotherapy, but the overall effect was not ideal. It is exciting to have this novel treatment method in this field, and it is also one of the most exciting abstracts at the 2022 ASCO annual meeting.
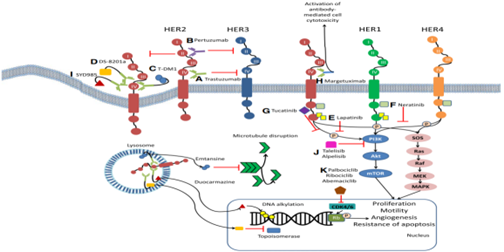
Mechanism of action of standard and new therapeutic HER2 oriented therapy [2]
In 2022 (as of August), Enhertu has been approved by FDA for three times:
1. On May 4, 2022, it was approved for the second line treatment of HER2 positive breast cancer (in December 2019, it was approved for the first time as the third line treatment of HER2 positive breast cancer);
2. On August 5, 2022, it was approved for second-line treatment of breast cancer with low HER2 expression (the first drug approved for breast cancer with low HER2 expression in the world) [3];
3.On August 11, 2022, FDA again approved Enhertu for second-line treatment of HER2 mutant non-small cell lung cancer (the first drug approved for HER2 mutant NSCLC in the world) [4], and also approved Life's Oncoming Dx Target Test (tissue) and GH's Guardant360 CDx (plasma) as its accompanying diagnosis. If HER2 mutation is not detected in the plasma ctDNA sample, the tumor tissue sample should be tested again. [5]

Figure 1[6]
Figure 2[7]
On August 11, the US FDA announced that Antibody Coupling Drug (ADC) Enhertu (DS-8201, T-DXd) has extended its indications for the treatment of patients with unresectable or metastatic non-small cell lung cancer (NSCLC) who have previously received a systematic treatment and carry an active HER2 mutation. Enhertu became the first drug for HER2 mutant non-small cell lung cancer.
Figure 3[7]

NCCN guidelines recommend targeted drugs for HER2 mutant NSCLC [8]
Reference
1.FDA approves first targeted therapy for HER2-low breast cancer. News release. FDA. August 5, 2022. Accessed August 14, 2022. https://bit.ly/3d4X2JQ
2.]CA CANCER J CLIN 2020;70:355–374
3.https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-regular-approval-fam-trastuzumab-deruxtecan-nxki-breast-cancer
4.https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-fam-trastuzumab-deruxtecan-nxki-her2-low-breast-cancer
5.https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-fam-trastuzumab-deruxtecan-nxki-her2-mutant-non-small-cell-lung
6.Guardant Health
7.FDA Official website
8.NCCN Lung Cancer Guidelines V3