Current location: Home > NEWS > Industry news
NEWS
PRODUCTS
Molecular mechanism and targeted therapy of low-grade serous ovarian carcinoma-part 1
News source: Release time:[2022-12-13]
part 1
Abstract
Epithelial carcinoma (EOC) is the most common malignant tumor of ovary, accounting for 80%~90%; Serous ovarian cancer (SOC) accounts for 70%~75% of EOC. Low grade serous ovarian cancer (LGSOC) is a relatively rare epithelial cancer, accounting for 6%~10% of serous ovarian cancer and 5% of all ovarian cancer. Its clinical and molecular biological characteristics are different from those of high-grade serous ovarian cancer (HGSOC). LGSOC is mostly found in young women, with slow growth and inert tumor, but it is also often in the progressive stage when it is found, and the 10-year survival rate is low (10%~20%). LGSOC is relatively insensitive to platinum based chemotherapy; They often carry mutations of KRAS and BRAF genes, and targeted therapies such as MEK inhibitors may play an important role [1-5].
Etiology and Molecular Mechanism
LGSOC is usually secondary to serous borderline tumor (SBOT). About 60% of LGSOC is related to SBOT [2], and it is also said that the correlation reaches more than 75% [3]. There is also a hypothesis that the fallopian tube epithelium is shed and planted on the surface of the ovary and peritoneum, thereby secondary to endometriosis of the fallopian tube, on this basis, atypical hyperplasia occurs, and then develops into SBOT and LGSOC[3].
SBOT develops into LGSOC through a series of gene mutations, including BRAF, KRAS, and ERBB2 gene mutations, which eventually lead to continuous activation of the MAPK pathway. LGSOC often carry KRAS (19%-55%) and BRAF (0-33%, average 5%) gene mutations, and may also have NRAS, USP9X and EIF1AX mutations, 9p deletion or CDKN2A/2B heterozygosity loss. Mutations in the KRAS gene may be an oncogenic driver of LGSOC progression and are also associated with tumor recurrence. MAPK is an important molecular pathway of LGSOC. In addition to gene mutations, deletion and fusion of MAPK pathway genes may also be related to the efficacy of MEK inhibitors. Some LGSOC patients may also have PI3K/AKT/mTOR pathway activation[3-4].
A larger LGSOC gene study (n = 71) analyzed 127 candidate genes from a whole-exome sequencing cohort to generate mutation and copy number variation data; immunohistochemistry (IHC) was performed, Estrogen receptor (ER), progesterone receptor (PR), p53, and CDKN2A status were assessed. Targeted sequencing identified mutations in RAS/RAF pathway genes (KRAS (26.7%), BRAF (12.6%), and NRAS (8.5%)) in 47% of cases, as well as USP9X (27%), MACF1 (11%), ARID1A ( 9%), NF2 (4%), DOT1L (6%), and ASH1L (4%) putative driver gene mutations. IHC showed frequent ER/PR positivity (85%), as well as CDKN2A protein loss (10%) and CDKN2A protein overexpression (6%). Loss or overexpression of CDKN2A is associated with shorter disease-specific survival (DSS). All samples were p53 wild type [5].
LGSOC cohort mutation spectrum[5]
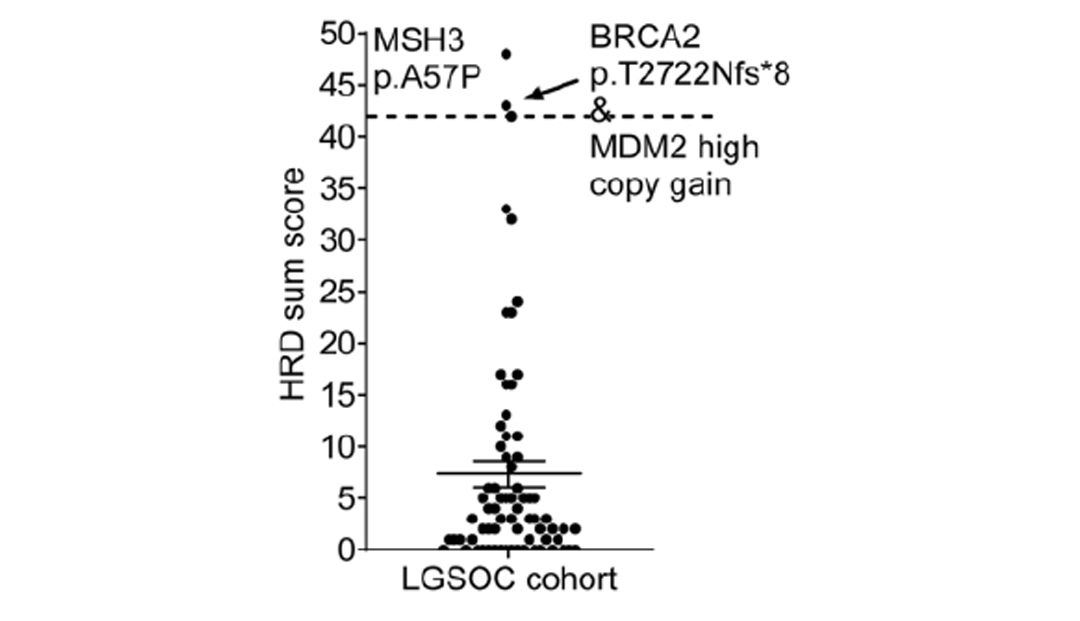
The study also analyzed homologous recombination repair deficiency (HRD) status. Unlike HGSOC, the average HRD score of LGSOC was only 3 (0 to 48 distribution), and only 3 cases with a score greater than 42 were judged as positive. A sample with HRD of 43 points had BRCA2 frameshift mutation (p.T2722Nfs*8) and copy number neutral loss of heterozygosity (LOH) at the same time. Studies have suggested that PARP inhibitors may benefit HRD-positive LGSOC patients, but more clinical studies are needed [5].
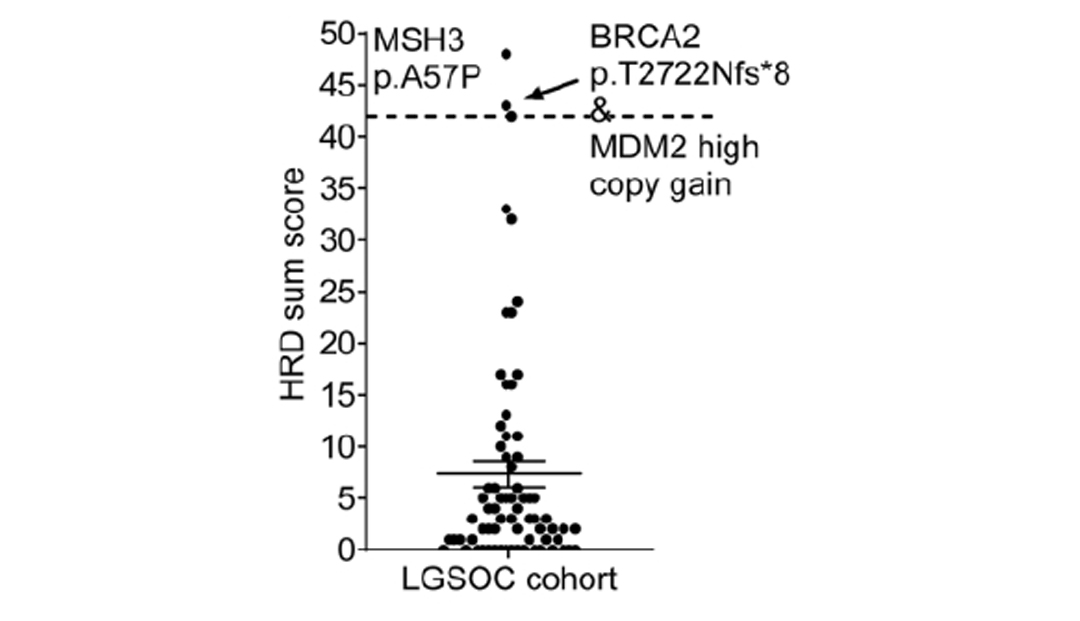
HRD Score Summary [5]
Histological Diagnosis and Differentiation
LGSOC is mainly diagnosed by pathological analysis of biopsy or surgical specimens. SOC histological grading adopts a two-level system, and the main grading basis is based on nuclear atypia and mitotic index: mild to moderate atypia, ≤12 mitoses per 10 high-power microscopes (HPF), diagnosed as LGSOC; with obvious nuclear atypia Sex, every 10 HPF>12 mitoses, diagnosed as HGSOC[3]. The clinical features are also different, and the specific features are shown in the table below.
Clinical features | LGSOC | HGSOC |
| median age of onset | Age 43~55 | Age 56~63 |
Proportion in SOC | 6%~10% | 90%-94% |
PFS | 19.5 month | 19.5month |
OS | 99.0 month | 57.0 month |
OS | 75.0% | 40.0% |
overall response rate to chemotherapy | 23.1% | 90.1% |
precancerous lesion | Papillary hyperplasia of fallopian tube epithelium | Serous intraepithelial carcinoma of fallopian tube epithelium |
Molecular biological features | KRAS,BRAF,ERBB2,NARS gene mutation | TP53 gene mutation, BRCA gene mutation and homologous recombination deficiency |
Clinical features of LGSOC and HGSOC [3]
Overview of Treatment[3]
Surgery is the main treatment for LGSOC, and chemotherapy or endocrine therapy is also the recommended treatment option. Patients with recurrent LGSOC have multiple options, including secondary cytoreductive surgery (SCRS), chemotherapy, endocrine/hormonal therapy, targeted inhibitors, and clinical trials. At the same time, molecular sequencing was performed to obtain somatic mutation profiles in order to determine the best therapeutic targets.
A retrospective study of the AGO database shows that the resistance rate of initial chemotherapy in LGSOC is 4% to 25%, and the chemotherapy regimen of most treatment options is analogous to that of HGSOC, that is, chemotherapy combined with platinum and paclitaxel after cytoreductive surgery (CRS) Program. Relapsed patients had a lower response rate to chemotherapy (3.7%), but due to the lack of prospective research support, chemotherapy is still the treatment of choice for recurrent LGSOC in addition to hormone therapy. The response of LGSOC to neoadjuvant chemotherapy is basically the same as that of initial chemotherapy, and the sensitivity of LGSOC to neoadjuvant chemotherapy is far less than that of HGSOC. Neoadjuvant chemotherapy is not recommended for advanced LGSOC.
In view of the low response rate to chemotherapy for newly diagnosed and relapsed LGSOC, postoperative endocrine therapy can be selected for patients with stage Ic and stage II-III, including Anastrozole, Letrozole, Exemestane, Leuprorelin, and Tamoxifen, the use of endocrine therapy in relapsed patients also has a certain effect.