Current location: Home > PRODUCTS > Digital PCR Series Products
PRODUCTS
(For Research Use Only)
Human epidermal growth factor receptor 2 (HER2) is an important prognostic factor for breast cancer. Breast cancer with HER2 overexpression have poor response to conventional chemotherapy and endocrine therapy, exhibit aggressive tumor invasion, shorter disease-free survival, and overall poor prognosis. Accurate detection and assessment of HER2 gene copy number in breast cancer and gastric cancer are crucial for clinical treatment and prognosis evaluation. HER2 testing results not only determine the suitability of targeted therapy for HER2 but also guide the selection of endocrine therapy, chemotherapy regimens, and prognosis assessment. Digital PCR detection results show high concordance with fluorescence in situ hybridization (FISH) and have advantages such as good accuracy, high precision, simple operational procedures, and objective results. The digital PCR detection method eliminates the need for manual interpretation, greatly overcoming the challenges associated with tumor heterogeneity in assessment.
THE RESULT OF HER2 OVEREXPRESSION
Normal HER2 expression
HER2 gene amplification leading to HER2 overexpression
Leading to tumor development
HER2 GENE TARGETING DRUG HERCEPTIN
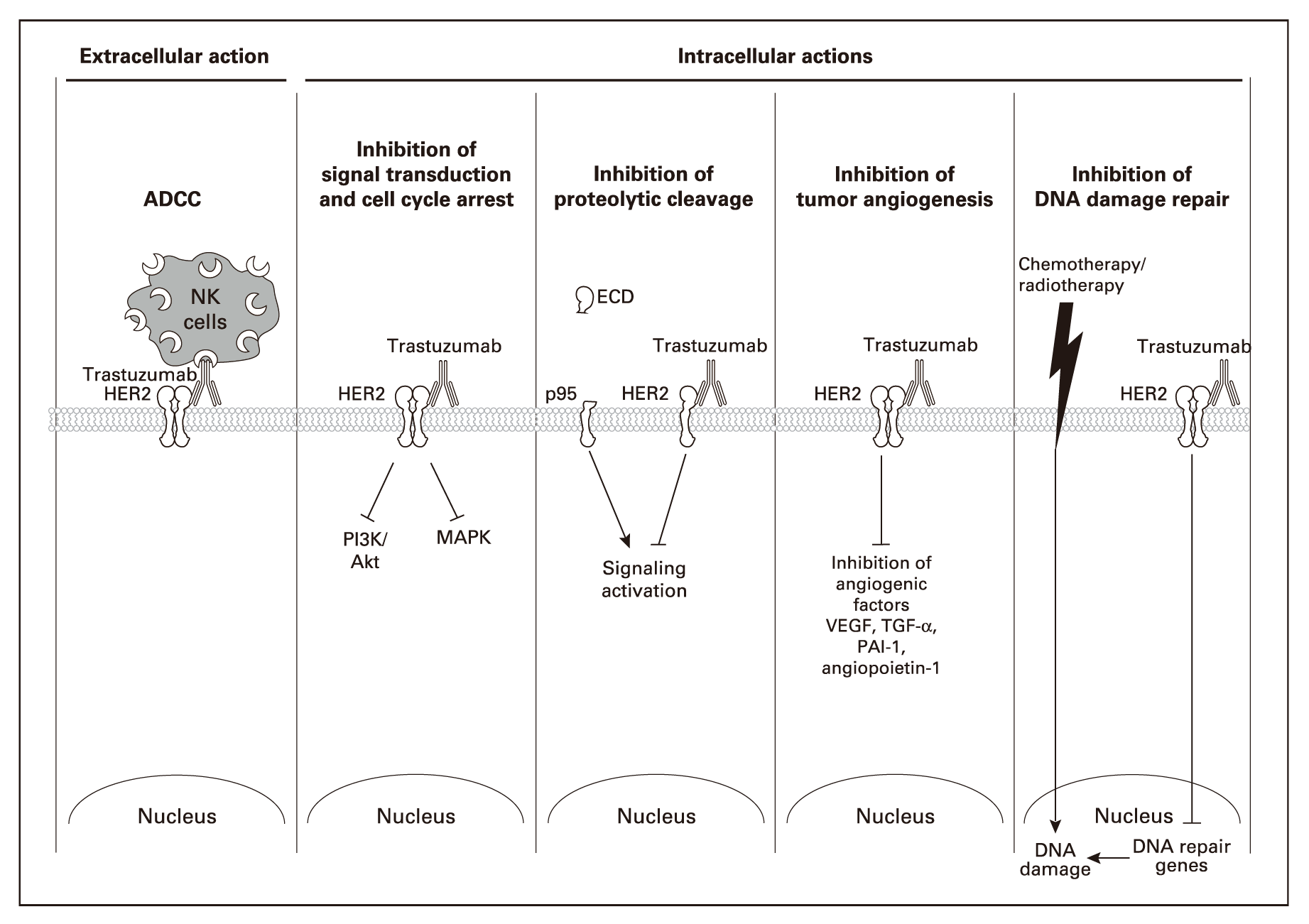
Herceptin was approved for the first time in 1998. As a monoclonal antibody drug, it can specifically inhibit the growth of cancer cells with HER2 oncogene amplification and greatly improve the prognosis of patients.
PRODUCT INFORMATION
| Product Name | Technology | Pack Size | Instruments Validated | Sample Type |
HER2 Gene Amplification Detection Kit | Digital PCR | 24 tests/kit 48 tests/kit | BIO-RAD:QX200 | Tumor tissue Peripheral blood Pleural effusion & Ascites |
DETECTION SIGNIFICANCE
1. It is used to detect HER2 gene amplification in patients with breast cancer, gastric cancer, colorectal cancer, endometrial carcinoma, etc., providing scientific evidence for the rational selection of drugs such as trastuzumab and pertuzumab in clinical practice.
2. It is applicable to patients with specific types of breast cancer, gastric cancer, colorectal cancer, endometrial carcinoma, etc.
FEATURES & ADVANTAGES
1. High Sensitivity: Can detect mutations with DNA sample content as low as 0.1%.
2. Accurate Quantification: Quantitatively detects gene copy number variations, enabling timely detection of disease progression and adjustment of treatment plans.
3. No Need For Internal Controls: Avoids false-negative results.
4. Simple And Fast: Testing can be completed within one working day in the hospital/laboratory setting.
DETECTION PROCESS
1. Nucleic Acid Extraction
2. Microdrop Preparation
3. Microdrop PCR
4. Data Analysis
5. Report