Current location: Home > PRODUCTS > Digital PCR Series Products
PRODUCTS
(For Research Use Only)
BACKGROUND
Non-small cell lung cancer (NSCLC) patients with EGFR mutations can achieve good therapeutic effects by using epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs) such as gefitinib, erlotinib, and osimertinib. However, primary/acquired resistance to EGFR-TKIs, such as gefitinib, erlotinib, and osimertinib, cannot be avoided. Among them, MET amplification is one of the causes of resistance to EGFR-TKIs, accounting for about 5% of the resistance reasons.
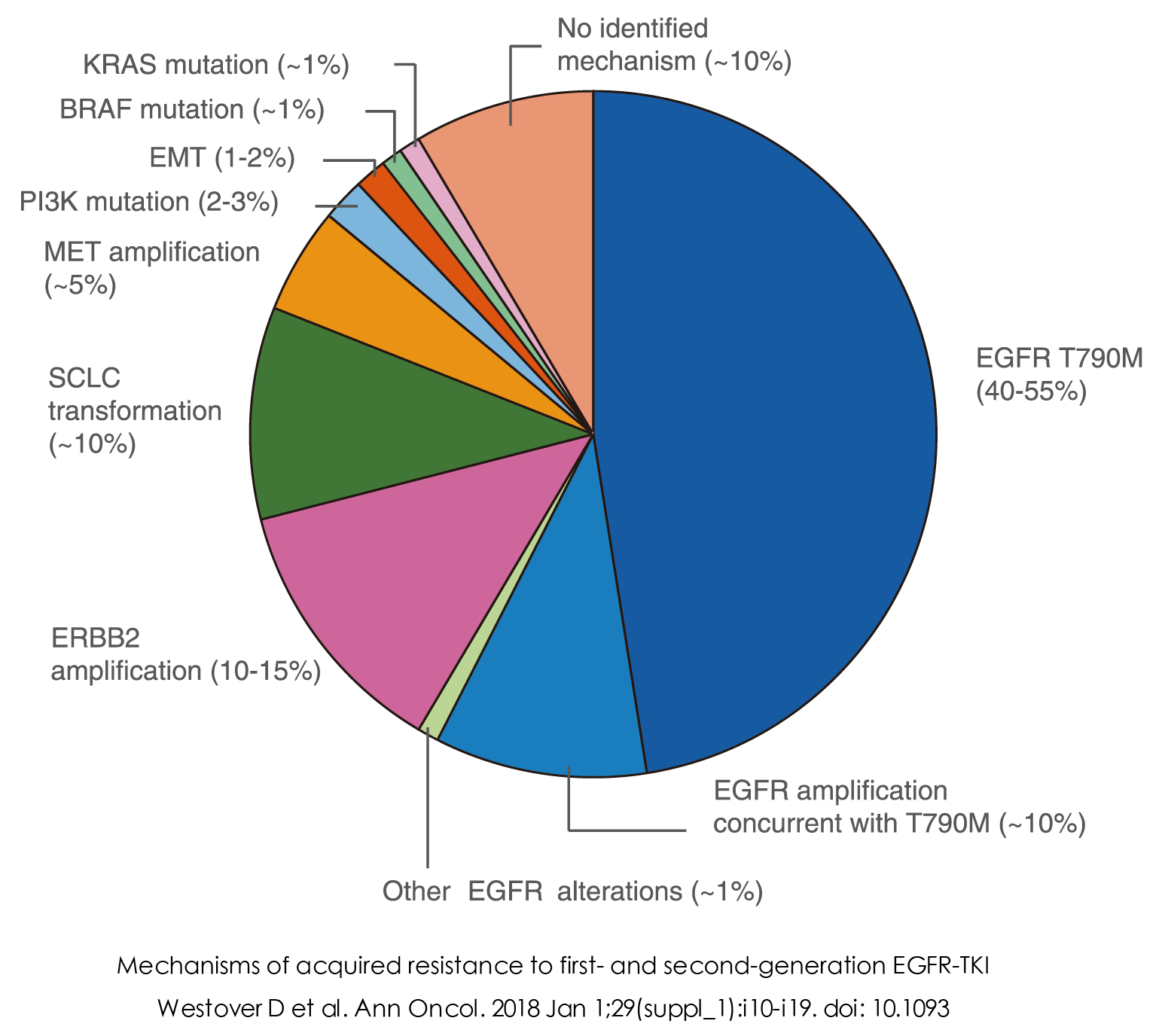
GENE INTRODUCTION
MET is an oncogene and one of the driver genes in various cancers. MET amplification activates the ERBB3 (HER3)-dependent activation of the PI3K pathway, which is specific to the EGFR/ERBB receptor family. The ultimate result is the promotion of cell transformation, cell invasion, cell proliferation, and cell cycle progression, among other effects. MET amplification can coexist with other oncogenic drivers, such as EGFR, ALK, and KRAS.
PRODUCT INFORMATION
| Product Name | Technology | Pack Size | Instruments Validated | Sample Size |
| MET Gene Amplification Detection Kit | Digital PCR | 24 tests/kit 48 tests/kit | BIO-RAD:QX200 | Tumor tissue Peripheral blood Pleural effusion &Ascites |
DETECTION SIGNIFICANCE
Before initiating treatment for NSCLC patients, detecting MET amplification helps match patients with appropriate targeted drugs based on genetic mutation information. This improves the specificity and effectiveness of treatment, ultimately achieving personalized therapy.
FEATURES & ADVANTAGES
1. High sensitivity: Can detect mutations with DNA sample content as low as 0.1%.
2. Accurate quantification: Quantitatively detects gene copy number variations, enabling timely detection of disease progression and adjustment of treatment plans.
3. No need for internal controls: Avoids false-negative results.
4. Simple and fast: Testing can be completed within one working day in the hospital/laboratory setting.
DETECTION PROCESS
1. Nucleic Acid Extraction
2. Microdrop Preparation
3. Microdrop PCR
4. Data Analysis
5. Report