Current location: Home > PRODUCTS > Real Time PCR Series Products
PRODUCTS
(CE-IVD)
MUTATIONS IN THYROID CANCER
The RET proto-oncogene is located at 10q11.2, is 60 kb in length, and contains 21 exons. After the RET gene is fused, the dimerization can be completed without ligands, resulting in the RET tyrosine kinase region, downstream MAPK、 PI3K/AKT and other signal pathways are continuously activated. It continues to drive cell proliferation, migration and differentiation etc., which will cause the occurrence of tumors. RET is one of the most common driver genes for papillary thyroid carcinoma (PTC).13 different oncogenic RET/PTC fusion proteins have been discovered [1]. Among them, RET/PTC1 accounts for about 60% of RET-related PTC, RET/PTC3 accounts for about 30%, and RET/PTC2 accounts for about 10%. The remaining RET/PTC family members are extremely rare [1].
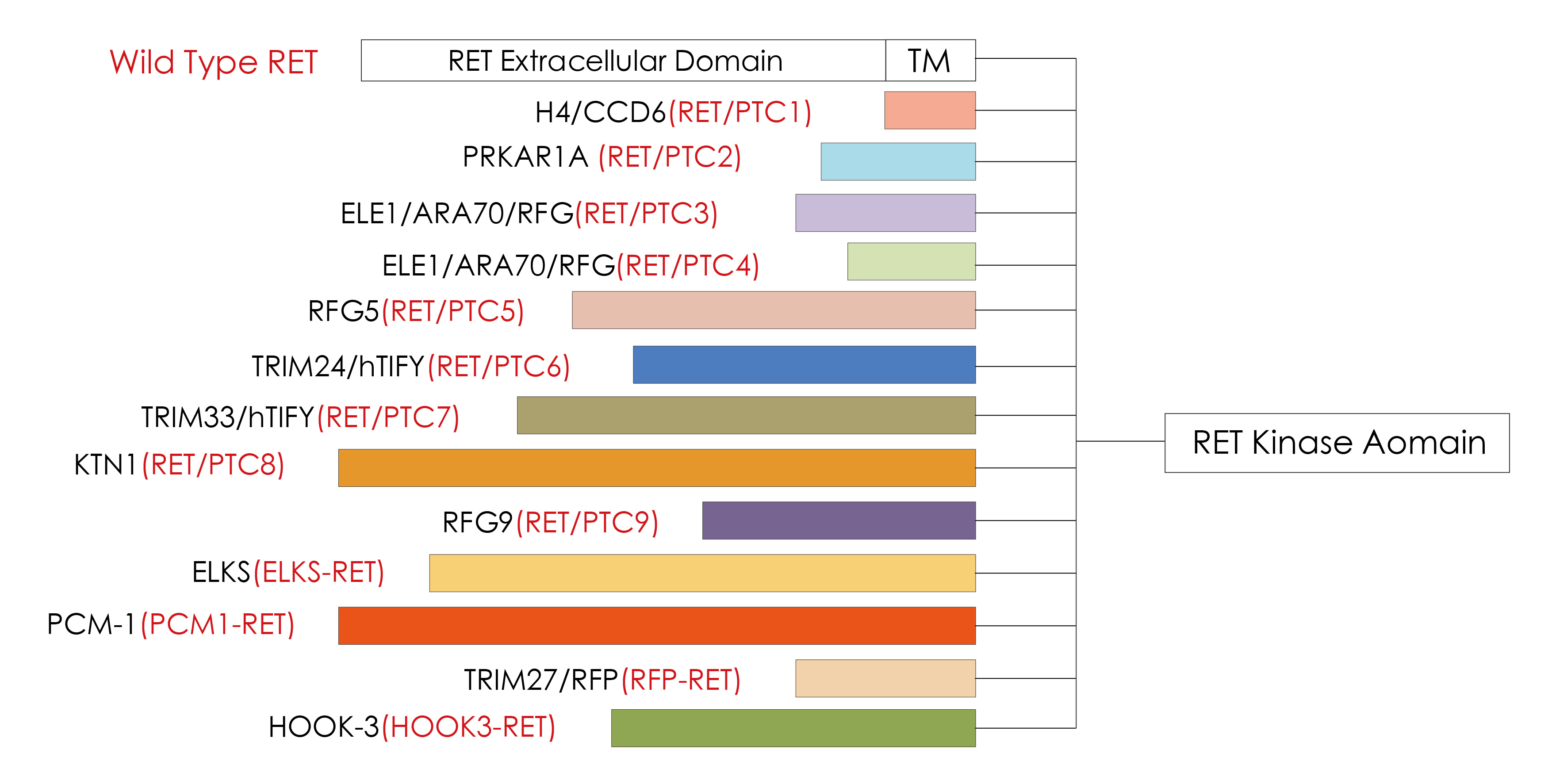
Knownmembers of RET/PTC fusion protein family [1]
RET FUSION AND TREATMENT OF THYROID CARCINOMA
The incidence of RET/PTC fusion in sporadic PTC is 15%-20%, and the malignant diagnosis rate for Bethesda III nodules is 60%. There is no false positive, which indicates that RET/PTC fusion is a very specific marker of PTC diagnosis [2], that can assist in the diagnosis of benign and malignant thyroid FNA samples.
RET fusion also has a suggestive effect on the efficacy of targeted therapy. In 2020, the FDA successively approved Selpercatinib and Pralsetinib for the systemic treatment of RET fusion-positive thyroid cancer patients, and approved RET fusion testing as a companion diagnosis.
DETECTED GENES
| Reaction Tube | Fusion Partner and Its exons | RET gene Exon | Reaction Tube | Fusion Partner and Its Exons | RET Gene Exon |
| RET-1 | CCDC6 E1 | E12 | RET-3 | PCM1 E29 | E12 |
| NCOA4 E8 | GOLGA5 E7 | ||||
| PRKAR1A E7 | HOOK3 E11 | ||||
| RET-2 | KIF5B E15 | KTN1 E29 | |||
| KIF5B E16 | RET-4 | NCOA4 E6 | |||
| KIF5B E22 | |||||
| KIF5B E23 |
PRODUCT INFORMATION
| Product Name | Technology | Pack Size | Instruments Validated | Sample Type |
| Thyroid Carcinoma RET Gene Fusions Detection Kit | Real-Time PCR assay | 20 Tests/Kit | ABI7500, ABI7300, ABI StepOne Plus, LightCycler480, Bio-Rad CFX96, etc. | Tumor tissue FNA |
DETECTION SIGNIFICANCE
1. FNA samples of thyroid nodules, coarse needle puncture samples or surgical resection samples for RET gene fusion detection can help assist in the diagnosis of PTC.
2. Patients with unresectable, refractory iodine, locally recurring or metastatic thyroid carcinoma should be tested for RET gene fusion status to help guide treatments.
FEATURES & ADVANTAGES
1. Accuracy and Reliability: Use pre-load PCR tube to effectively avoid cross-contamination.
2. High Sensitivity: Sensitivity can detect as low as 100 copies of fusion mutations in RNA.
3. Comprehensive Coverage: Covers the 12 most common RET fusion variants in thyroid cancer.
4. Great Versatility: Validated on the most common qPCR machines with stable results.
DETECTION PROCESS
1. Nucleic Acid Extraction
2. Set up qPCR
3. Amplification
4. Data Analysis
[1] Cancer. 2015 Jul 1;121(13):2137-46.
[2] Guangdong Expert Consensus on Gene Detection and Clinical Application of Thyroid Cancer (2020 Edition)