Current location: Home > PRODUCTS > Real Time PCR Series Products
PRODUCTS
(CE-IVD/NMPA)
GENE MUTATION AND TUMOR
Epidermal growth factor receptor (EGFR) is a receptor tyrosine kinase, expressed in 50% of non-small cell lung cancer (NSCLC) [1], which is closely related to the occurrence and development of tumors. EGFR is the most frequently mutated driver gene in NSCLC patients in East Asian population, with a mutation frequency of 38.4%, among which East Asian female NSCLC patients have a mutation frequency of 51.1% [2]. EGFR gene mutation is the most important predictor of the efficacy of EGFR Tyrosine Kinase Inhibitor (TKI) and is a prerequisite for clinically determining whether patients can use EGFR-TKI therapy. Both the National Comprehensive Cancer Network (NCCN) and the Chinese Society of Clinical Oncology (CSCO) guidelines include EGFR mutation detection as a category 1 recommendation [3][4].
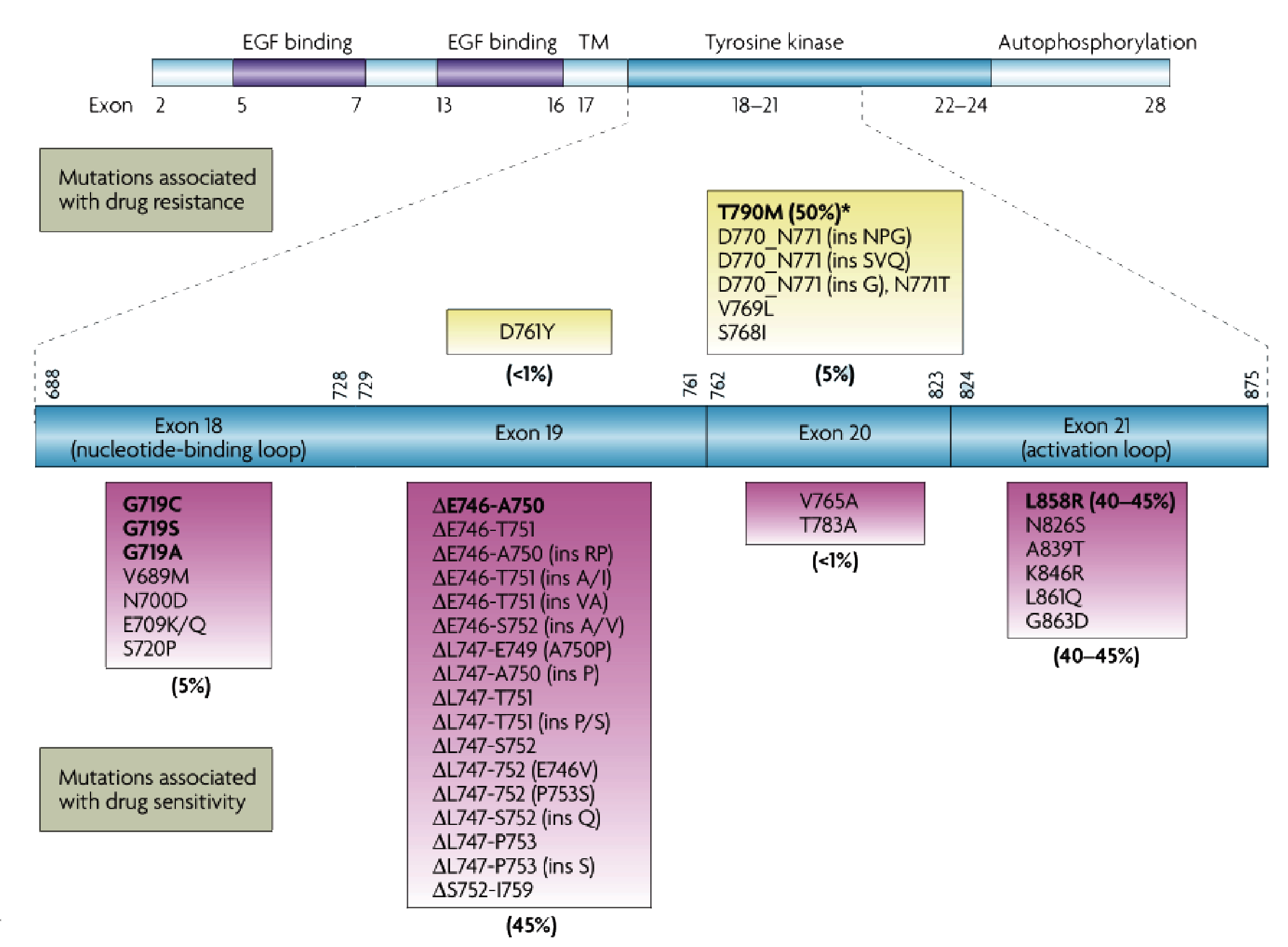
Common mutations in the EGFR gene [1]
COMMON MUTATIONS
The carcinogenic mutations of EGFR mostly occur in exons 18-21, of which 19 del and L858R account for 85% to 90% of EGFR mutations [1] which are also the most common EGFR-TKI sensitive mutations. The T790M mutation suggests resistance to the first and second generation of EGFR-TKI while sensitive to the third generation of EGFR-TKI. For 20 ins mutation,which is not sensitive to traditional EGFR-TKI, suggesting the use of EGFR 20 ins inhibitor.
DETECTED GENES
| Gene | Covering Exons | Medication |
| EGFR | 19 del, L858R | EGFR-TKI |
| G719X, S768I, L861Q | EGFR-TKI(Except for Befotertinib) | |
| T790M | Osimertinib,Almonertinib,Furmonertinib, Befotertinib | |
| 20 ins | Amivantamab,Mobocertinib |
PRODUCT INFORMATION
| Product Name | Core Technology | Pack Size | Instruments Validated | Sample Type |
EGFR Gene Mutation Detection Kit | PAP-ARMS® | 10 tests/kit | ABI7500, ABI7300, ABI StepOne Plus, LightCycler480, Bio-Rad CFX96, etc. | Tumor tissue Peripheral blood Pleural effusion |
DETECTION SIGNIFICANCE
1. EGFR mutation detection in resectable stage IB-IIIA NSCLC patients to guide adjuvant targeted therapy.
2. The detection of EGFR mutation for unresectable stage III and IV NSCLC patients, can help guide the treatments according to molecular classification.
3. For patients with EGFR-TKI resistance, it is recommended to perform another biopsy for EGFR gene mutation detection.
FEATURES & ADVANTAGES
1. Accuracy and Reliability:Use pre-load PCR tube to effectively avoid cross-contamination.
2. High Sensitivity: Sensitivity can reach as low as 1%in 10 ng DNA.
3. Ease of Use: Based on technology PAP-ARMS, one step detection in 90 mins.
4. Great versatility:Validated on the most common qPCR machines with stable results.
DETECTION PROCESS
1.Nucleic Acid Extraction
2.Set up qPCR
3.Amplification
4.Data Analysis
[1] Nat Rev Cancer. 2007 Mar;7(3):169-81.
[2] Oncotarget. 2016 Nov 29;7(48):78985-78993.
[3] NCCN Clinical Diagnosis and Treatment Guidelines NSCLC 2023 V3
[4] CSCO NSCLC Diagnosis and Treatment Guidelines 2023