Current location: Home > PRODUCTS > Digital PCR Series Products
PRODUCTS
(CE-IVD)
INTRODUCTION OF EGFR
Epidermal growth factor receptor (EGFR) is a receptor tyrosine kinase, expressed in 50% of non-small cell lung cancer (NSCLC) [1], which is closely related to the occurrence and development of tumors. The carcinogenic mutations of EGFR mostly occur in exons 18-21, of which 19 del and L858R account for 85% to 90% of all EGFR mutations [1], which are also the most common EGFR-TKI sensitive mutations. The T790M mutation suggests resistance to the first and second generation of EGFR-TKIs while sensitive to the third generation of EGFR-TKI. Both the National Comprehensive Cancer Network (NCCN) and the Chinese Society of Clinical Oncology (CSCO) guidelines have included EGFR mutation detection as a category 1 recommendation [2] [3]. The guidelines also clearly stated that "When tumor tissue cannot be obtained or can be obtained for small amounts ,EGFR mutation detection can be performed through peripheral blood /tumor DNA (cf/ctDNA) [3]".
INTRODUCTION OF ctDNA
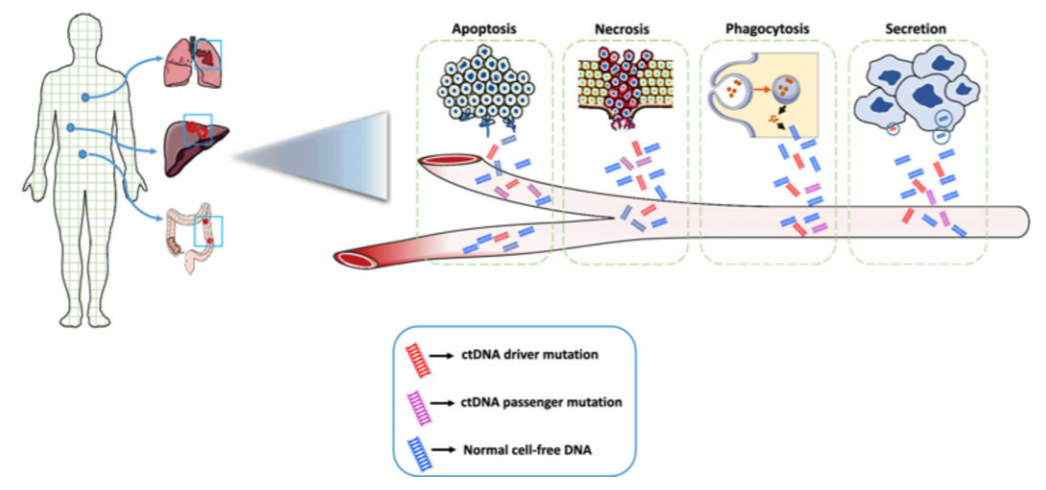
ctDNA is released into blood vessels by tumor cells or circulating tumor cells through apoptosis, necrosis, phagocytosis, or active secretion. It can reflect the real-time tumor load in the body, dynamically monitor the efficacy of drugs, and predict disease changes early. ctDNA has become a dynamic biomarker based on plasma, which plays an important role in the fields of early tumor diagnosis, molecular typing, targeted drug selection, and monitoring of minimal residual disease. Since the content of ctDNA is usually less than 1% of the total cfDNA of an individual [4], screening ctDNA from a large amount of cfDNA requires high detection sensitivity. Liquid biopsy technology based on digital PCR makes its possible to detect low concentrations of ctDNA. ctDNA is released into the blood[5].
DETECTED GENE
| Gene | Mutation | Drug Indication |
| EGFR | L858R | EGFR-TKI |
| T790M | Osimertinib, Almonertinib, Furmonertinib,Befotertinib |
PRODUCT INFORMATION
| Product Name | Technology | Pack Size | Instruments Validated | Sample Type |
| EGFR Gene T790M Mutation Detection Kit(CE-IVD) | Digital PCR | 24 Tests/Kit, 48 Tests/Kit | Bio-Rad QX200 | Tumor tissue, Peripheral blood, Pleural and ascites |
EGFR Gene L858R Mutation Detection Kit(RUO) |
DETECTION SIGNIFICANCE
1. EGFR mutation detection in operable stage IB-IIIA NSCLC patients to guide adjuvant targeted therapy.
2. It is recommended to detect EGFR gene status before first line treatment for patients with inoperable stage III and IV NSCLC to guide the treatments according to molecular classification. If tissue samples are not available, circulating tumor DNA can be considered for testing.
3. For patients with EGFR-TKI resistance, EGFR T790M gene mutation detection is recommended. If tissue samples are not available, ctDNA can be considered for testing.
4. Regularly monitor T790M resistance mutations after using EGFR-TKI drugs.
FEATURES & ADVANTAGES
1. High Sensitivity: Sensitivity can detect as low as 0.1% mutation in DNA.
2. Precise Quantification: Quantitative detection of gene mutation abundance, timely detection of disease progression, and adjustment of treatment plans.
3. No internal reference needed: Avoid false negative.
4. Fast Results: Complete detection process in 1 working day.
DETECTION PROCESS
1.Nucleic Acid Extraction
2.Microdrop Preparation
3.Microdrop PCR
4.Data Analysis
5.Report
[1] Nat Rev Cancer. 2007 Mar;7(3):169-81.
[2] NCCN Clinical Diagnosis and Treatment Guidelines NSCLC 2023 V3
[3] CSCO NSCLC Diagnosis and Treatment Guidelines 2023
[4]NatRevClinOncol.2020Dec;17(12):757-770.
[5]MolDiagnTher.2019Jun;23(3):311-331