Current location: Home > PRODUCTS > Next Generation Sequencing Series Products
PRODUCTS
Dapreen®
Next Generation Sequencing
(For Research Use Only)
With the effective control of acute infectious diseases and the extension of human average lifespan, cancer has become one of the major diseases seriously endangering human health, with aging accelerating the incidence and treatment pressure, especially for tumors, including cancer (similar to malignant tumors). Cancer is one of the top two leading causes of death for residents aged 30-69 in most countries. An important tool throughout the entire cancer management lifecycle is cancer molecular testing. Depending on the type, combination, and purpose of the detected biomarkers, it provides essential information about the occurrence, heterogeneity, progression, and prognosis of the disease, enabling effective intervention and treatment.
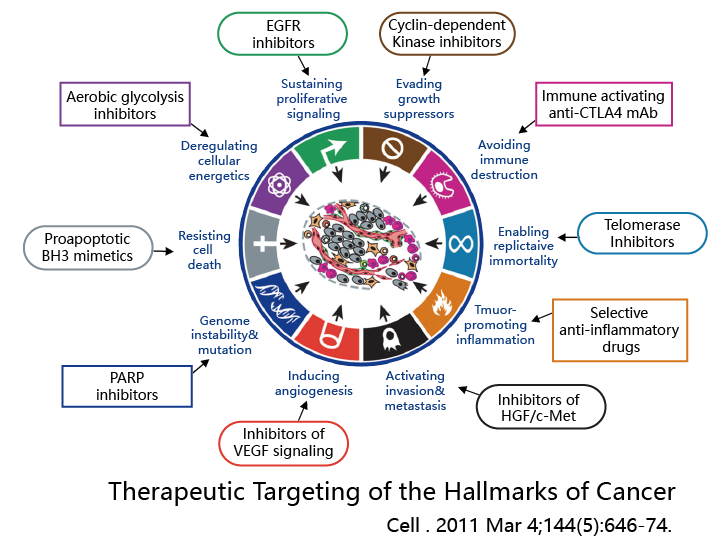
Tumor driver genes can provide insights into the progression of cancer and can be used to assess prognosis, recurrence, and metastatic risks. Genes related to drug response can help guide medication choices, offering information on drug sensitivity or resistance. Understanding the genetic status of a tumor forms the foundation for developing treatment plans. By selecting the appropriate genes, it's possible to achieve more precise cancer treatment, ensuring the maximum benefit from clinical therapy.
DETECTION CONTENT
All-in-one test for 175 cancer-related genes
Pan Solid Tumor Sequencing Panel(175 genes) is designed to identify all classes of actionable genomic alterations, including SNV,SNP, CNV, InDels, and Fusions, across a total of 175 cancer-related genes.
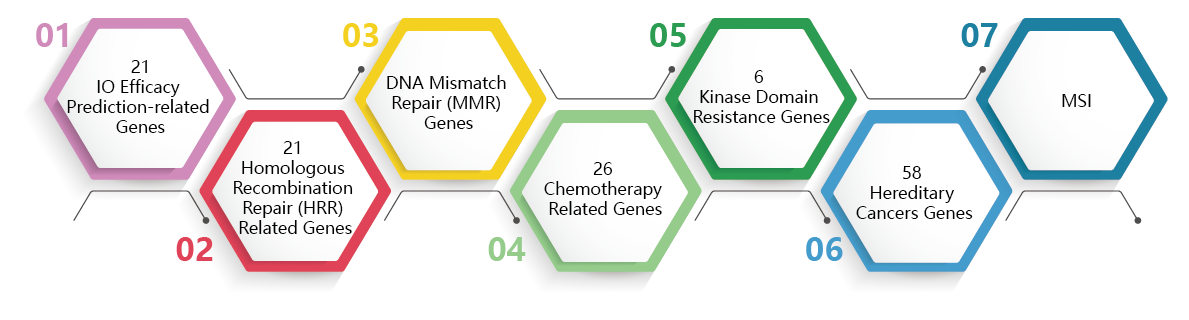
KEY BIOMARKERS
PRODUCT INFORMATION
Product Name | Core Technology | Pack Size | Instruments Validated | Sample Type |
Pan Solid Tumor Sequencing Panel Assay (175 genes, for tissue) | Probe Capture + Next Generation Sequencing | 24 Tests/kit | Illumina MGISEQ | Somatic: tumor tissue samples or cell-free DNA, pleural and ascites fluid Germline: peripheral blood |
Pan Solid Tumor Sequencing Panel Assay (175 genes, for blood) |
Pan Solid Tumor Sequencing Panel Assay(175 genes) includes DNA library construction assay , Pan Solid Tumor Sequencing Panel(175 genes) capture assay and SG Pure Beads.
DETECTION SIGNIFICANCE
1. Before undergoing targeted therapy, patients with malignant tumors can undergo genetic testing to assist clinical doctors in assessing the patient's sensitivity to the medication and predicting their prognosis.
2. Comprehensive evaluation of the benefits of PD-1/PD-L1 immunotherapy includes immune therapy markers such as MMR/MSI, as well as IO efficacy prediction-related genes.
3. SNP related to chemotherapy drugs are used to predict the efficacy and side effects of chemotherapy, aiding clinical doctors in formulating appropriate treatment plans.
4. For patients who develop drug resistance, experience recurrence, or undergo metastasis, genetic testing can be repeated to clarify the resistance mechanisms, assisting clinical doctors in creating personalized treatment plans.
5.Testing for HRR genes like BRCA1/2 can indicate some genetic risk and expand the population that benefits from PARP inhibitors.
ADVANTAGE & FEATURE
1. Comprehensive Coverage: This testing approach covers all the genes that hold significant clinical relevance in the diagnosis and treatment of malignant tumors, including those related to targeted therapy, immunotherapy, efficacy prediction for chemotherapy, assessment of toxicity and tolerance, and the diagnosis of hereditary cancers.
2. High Sensitivity: It employs probe capture and NGS for deep sequencing, minimizing the chances of missing important information and maximizing the insights related to medication.
3. Strict Quality Control: Quality control measures are implemented at various stages, including nucleic acid extraction, library preparation and data analysis, ensuring rigorous quality standards are maintained.
DETECTION PROCESS
1.Nucleic Acid Extraction
2.Library Preparation (3.5 hours total time)
3.Sequencing
4.Auto-data Analysis
5.Report