Current location: Home > NEWS > Industry news
NEWS
PRODUCTS
CheckMate-816 Research
News source: Release time:[2023-02-24]
Introduction: Professor Wu Yilong- Guangdong Provincial People's Hospital and Guangdong Lung Cancer Institute pointed out that the three key words in the field of lung cancer in 2022 are: minimal residual disease (MRD), CheckMate816 and KRAS. Today,let we talk about CheckMate816 research.
For Opdivo, 2022 is destined to be an extraordinary year, the Nivolumab and platinum-doublet chemotherapy has been successively approved by the CSCO Guidelines for the Clinical Application of Immune Checkpoint Inhibitors 2022", and NCCN. It has become the only NSCLC neoadjuvant systemic therapy recommended by the above-mentioned guidelines.

The update of these guidelines is based on a large global clinical study code-named CheckMate-816. Checkmate 816 is the first published global multi-center phase III clinical study on neoadjuvant immunotherapy for lung cancer, aiming to compare the efficacy and safety of preoperative Nivolumab
+ chemotherapy and chemotherapy alone.
Research design
Clinical Trial Design: An international, phase 3, randomized, openlabel trial examined the efficacy and safety of neoadjuvant nivolumab plus chemotherapy, as compared with chemotherapy alone, in adult patients with stage IB to IIIA NSCLC.
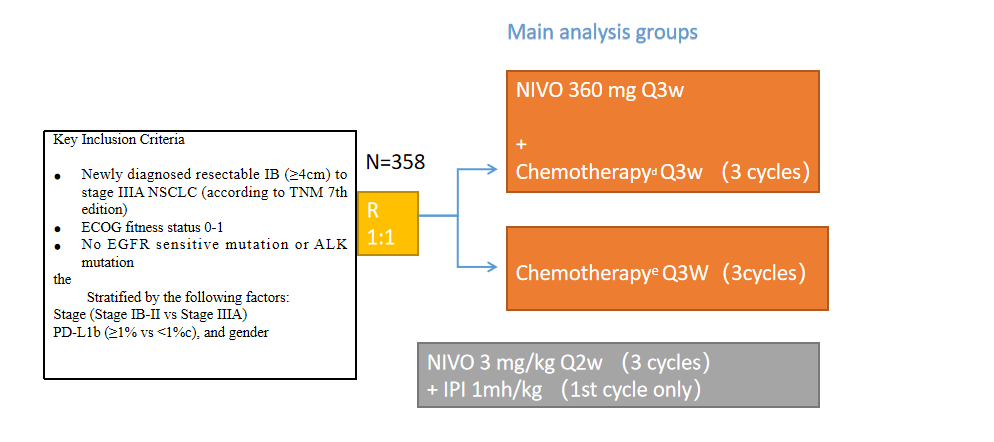
Checkmate-816 is a global multicentre, open-label, phase III randomised controlled study.
Inclusion criteria: IB-IIIA (7th TNM staging system) NSCLC patients were randomly assigned to receive preoperative Nivolumab (360mg) + chemotherapy (3 cycles/q3w) or chemotherapy (3 cycles/q3w) in a 1:1 ratio.
Main research endpoints: pCR, EFS. Secondary study endpoints: MPR, OS, time to death or distant metastasis.
The study excluded patients with EGFR or ALK mutations, and positive PD-L1 expression was not required.
A total of 358 patients were enrolled in the study, 179 of them entered the Nivolumab+chemotherapy group, and 179 of them entered the chemotherapy alone group. 176 of the two groups were successfully treated, and the baseline balance of the two groups was balanced.
Study results
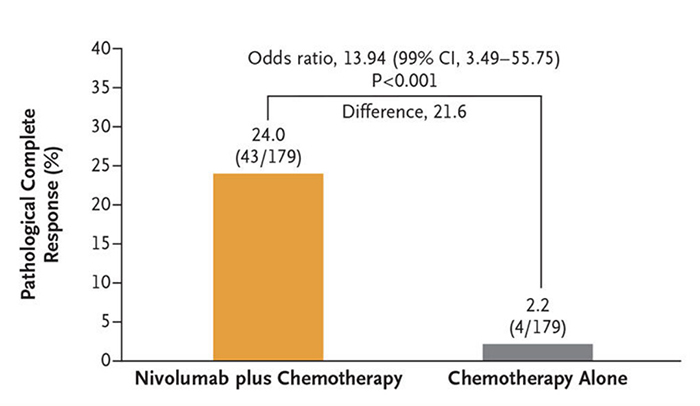
Primary study endpoint - pCR
pCR rate in the neoadjuvant immunotherapy + chemotherapy group: 24%
pCR rate in the chemotherapy alone group: 2.2%
10-fold increase in pCR rate in the neoadjuvant immunotherapy + chemotherapy group relative to the chemotherapy alone group
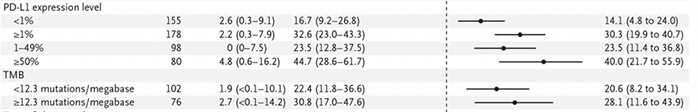
In the subgroup analysis, the pCR benefit of patients with PD-L1 ≥ 1% is more obvious, and the pCR benefit of the two groups with different tumor mutation burden (TMB) is relatively close, which suggests that the expression level of PD-L1 or the pCR gain more effective predictors.
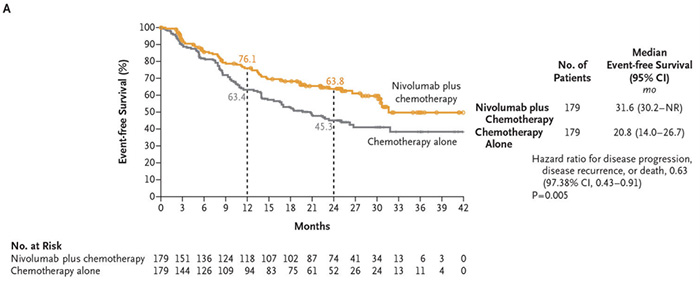
Primary study endpoint—EFS
neoadjuvant immunotherapy + chemotherapy group: 31.6 months
Chemotherapy alone group: 20.8
neoadjuvant immunotherapy + chemotherapy can prolong the EFS of patients by 10.8 months, and reduce the risk of disease progression, recurrence or death by 37% (HR 0.63; 97.38% CI, 0.43-0.91; P=0.005).
In subgroup analysis, patients with stage IIIA (HR 0.54 vs. 0.87), patients with PD-L1 ≥ 1% (HR 0.41 vs. 0.85) and patients with non-squamous cell carcinoma (HR 0.50 vs. 0.77) benefited significantly.
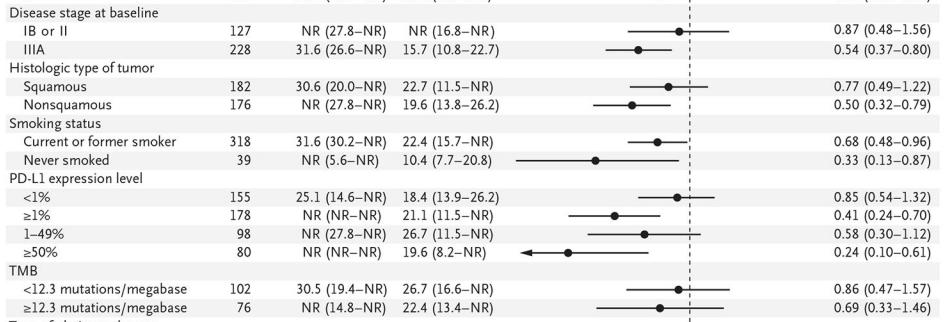
Secondary Study Endpoint—OS
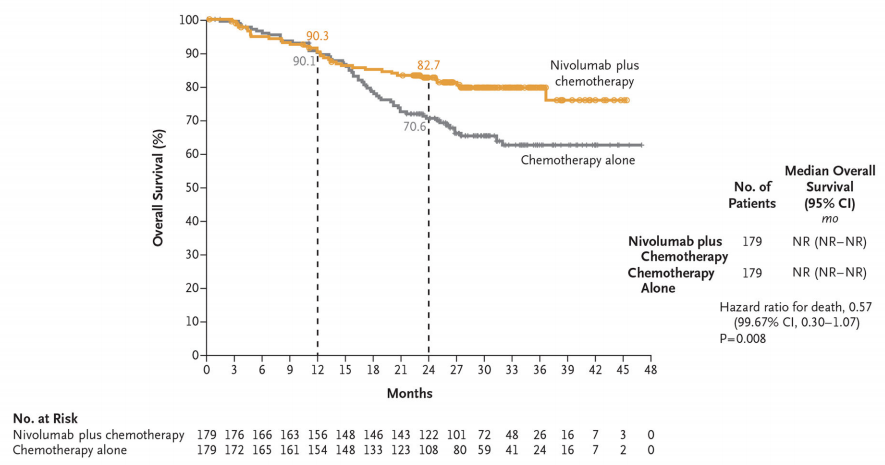
The overall survival data is not yet mature, and the preliminary analysis found that the trend of OS benefit is obvious (HR 0.57, 99.67%CI, 0.30-1.07).
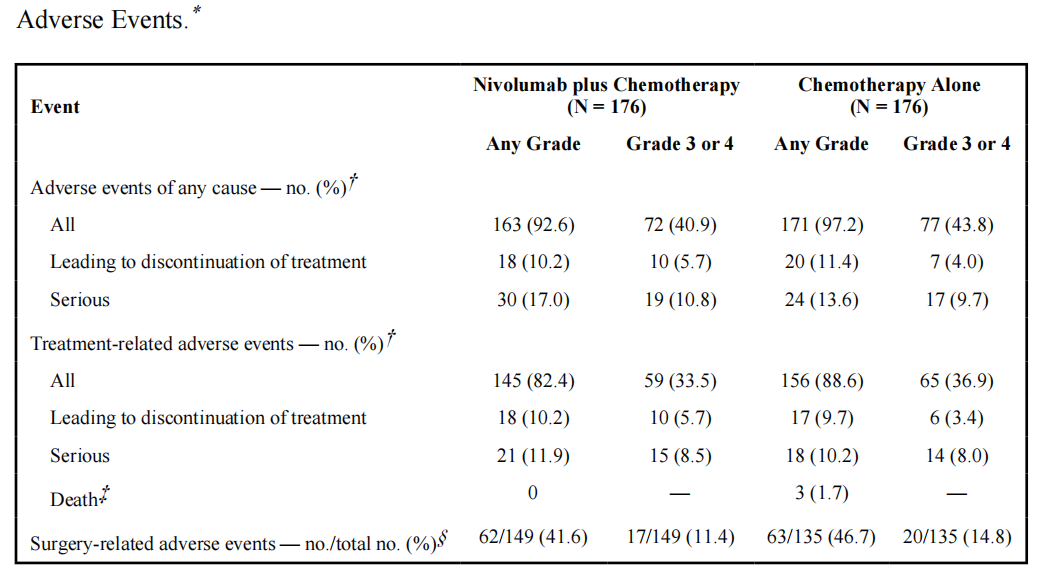
Safety: The incidence of grade 3 or 4 treatment-related adverse events was similar in the two groups;
SpaceGen overall solution for the PD-L1 detection of gene expression
Product name | Detection Assay | Pack size | Instrument Validated | Sample Type |
Human PD-L1 gene expression level detection | Digital PCR | 24 Tests/kit 48 Tests/kit | Bio-Rad:QX200 | Tumor tissue |
References:
[1].Forde PM et al. Neoadjuvant Nivolumab plus Chemotherapy in Resectable Lung Cancer[J]. The New England journal of medicine, 2022, 386: 1973-1985.
[2].Chinese Society of Clinical Oncology Guidelines Working Committee, "Chinese Society of Clinical Oncology (CSCO) Clinical Application Guidelines for Immune Checkpoint Inhibitors 2022".
[3].National Comprehensive Cancer Network 2022, Non-Small Cell Lung Cancer, Version 5. 2022, Sep. 26, 2022.