Current location: Home > NEWS > Industry news
NEWS
PRODUCTS
Screening and Molecular Typing of Gastric Cancer
News source: Release time:[2023-03-06]
01/ Worldwide gastric cancer
Stomach cancer
Stomach cancer remains an important cancer worldwide and is responsible for over one million new cases in 2020 and an estimated 769,000 deaths (equating to one in every 13 deaths globally), ranking fifth for incidence and fourth for mortality globally Rates are 2-fold higher in men than in women. In men, it is the most commonly diagnosed cancer and the leading cause of cancer death in several South Central Asian countries, including Iran, Afghanistan, Turkmenistan, and Kyrgyzstan . Incidence rates are highest in Eastern Asia ( Japan and Mongolia, the countries with the highest incidence in men and women, respectively) and Eastern Europe,18 whereas rates in Northern America and Northern Europe are generally low and equivalent to those seen across the African regions. [1]
02/Factors affecting gastric cancer
The main risk factors for gastric cancer include.
(i) Having the following diseases: Helicobacter pylori (Hp) infection, chronic atrophic gastritis, pernicious anaemia, intestinal metaplasia, gastric polyps, familial adenomatous polyposis, hereditary non-polyposis colorectal cancer.
(ii) Family history of gastric cancer.
(iii) History of gastric surgery.
④ Poor lifestyle and dietary habits, such as smoking, alcohol consumption, high salt diet, excessive intake of smoked food, low intake of fruit and vegetables, etc.
⑤ Age > 40 years.
⑥The incidence of gastric cancer is about twice as common in men as in women. The pathogenesis of gastric cancer is not well understood and may be caused by a combination of factors such as genetic factors, diseases and lifestyle habits. Among them, Hp infection has the most significant impact in the development of gastric cancer.
Adequate intake of vegetables and fruits is a protective factor against gastric cancer.
Familial aggregation is present in 5%-10% of gastric cancer patients. Common hereditary gastric cancers include HDGC, familial intestinal gastric cancer (FIGC), gastric adenocarcinoma and proximal polyposis of the stomach (GAPPS). polyposisof the stomach (GAPPS). In addition, Li-Frau⁃meni syndrome, familial adenoma⁃tous polyposis (FAP), Peutz-Jeghers syn⁃drome (PJS), Lynch syndrome, hereditary breast-ovarian cancer HBOCS, MUTYH-associated polyposis (MAP), juvenile polyposis syndrome (JPS), etc. Genetic diseases can also be combined with gastric cancer.
People with a family history of hereditary gastric cancer should be screened and assessed for genetic risk of gastric cancer as early as possible, regardless of age.
03/Molecular features of gastric cancer
As part of the Cancer Genome Atlas (TCGA) project, in 2014 TCGA published a comprehensive molecular assessment of 295 primary gastric adenocarcinomas. Blood or tissue samples were used to classify gastric cancers into four molecular subtypes using six molecular platforms: array-based somatic copy number analysis, whole exome sequencing, array-based DNA methylation profiling, messenger RNA sequencing, microRNA (miRNA) sequencing and reverse phase protein array (RPPA): EBV-positive (EBV), microsatellite unstable (MSI), genomically stable (GS), and chromosomally unstable (CIN). The staging strategy was to first classify tumours according to EBV positivity, then according to MSI status, and the remaining tumours were classified as genomically stable or chromosomally unstable according to the degree of aneuploidy. Preliminary outcome data from this cohort did not reveal differences in survival between the four subgroups nor were systematic differences observed between the Eastern and Western cohorts.
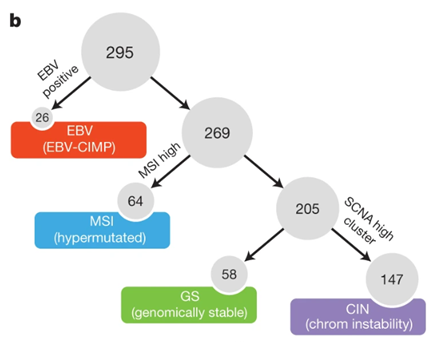
▲▲Staging strategy for TCGA gastric cancer[2]
①EBV-positive (EBV): This type accounts for about 9% of cases. Most EBV-positive tumors are found in the fundus or body of the stomach and tend to be male. EBV-positive tumors have a higher incidence of DNA hypermethylation than any of the cancers reported by TCGA. 80% of EBV-positive types have non-silent PIK3CA mutations with a more dispersed mutation site than EB-negative tumors. JAK2 amplification,CD274 (PD-L1) and PDCD1LG2 (PD-L2) were overexpressed, suggesting PD-L1/2 inhibitors and JAK2 inhibitors as potential therapeutic targets. Few TP53 mutations were present.
②Microsatellite instability type (MSI): this type accounts for about 22% of cases. It is prevalent in the gastric sinus or pylorus, mostly in women and elderly patients, and is generally characterized by high mutation rate and hypermethylation (including MLH1 promoter methylation), usually with PIK3CA,ERBB3, ERBB2 and EGFR mutations; there is no BRAF V600E mutation, which is commonly found in MSI colorectal cancer.
③Genomic stable type (GS): this type accounts for about 20% of cases. The age at first diagnosis is low (median age 59 years), and it is mostly Lauren diffuse type. Rich in CDH1, RHOA gene mutations and CLDN18-ARHGAP fusions, CDH1 germline mutations are the basis of hereditary diffuse gastric cancer (HDGC), however germline analysis revealed only two CDH1 gene polymorphisms and neither is the causative form of hereditary diffuse gastric cancer. When in the active GTP-bound form, RHOA controls actin-myosin-dependent cell contractility and cell motility and activates STAT3 to promote tumorigenesis via multiple effectors (including ROCK1, mDIA, and protein kinase N), and RHOA is almost exclusively in genetically stable tumors and is a hallmark of diffuse tumors. In genetically stable tumors RHOA mutations and CLDN18-ARHGAP6 or ARHGAP26 fusions are mutually exclusive.
④Chromosomal instability (CIN): this type accounts for approximately 50% of cases, is mostly male and has an older age of onset, often occurs at the gastroesophageal junction and cardia, and is mostly of the Lauren intestinal type. It shows significant aneuploidy and focal amplification of receptor tyrosine kinases. This type is often associated with elevated EGFR phosphorylation due to EGFR (pY1068) amplification and TP53 mutations, with frequent TP53 mutations and chromosomal heteroploidy. A large number of genomic amplifications of RTKs were also identified, many of which can be blocked by therapeutic agents currently in use or under development. In addition, frequent amplification of cell cycle regulatory genes (CCNE1, CCND1 and CDK6) suggests potential therapeutic targets for the inhibition of cyclin-dependent kinases.
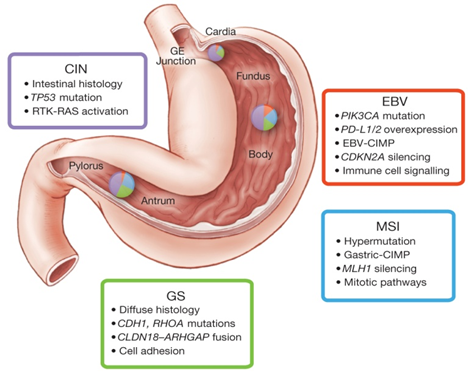
▲Main characteristics of the different subtypes[2]
In addition to TCGA typing, three major subtypes were identified at Duke National University School of Medicine in Singapore in 2013: proliferative, metabolic, and mesenchymal. The Asian Gastric Cancer Research Group (ACRG) proposed in 2015 to classify gastric cancer into 4 molecular subtypes: MSI type, MSS/EMT type, MSS/TP53 inactivated type (MSS/TP53- type), and MSS/TP53+ type. Also in 2015 Chinese scholars proposed to classify gastric cancer into two subtypes with high level of clonal heterogeneity (high-clonality) and low level of clonal heterogeneity (low-clonality) [3-5]. The molecular typing system of gastric cancer still needs to be improved to more accurately guide clinical practice.
Gastric cancer is a highly heterogeneous tumor that is strongly influenced by genotype. Research and classification at the molecular level can lead to more scientific management and personalized treatment plans for gastric cancer patients to achieve the goal of precise treatment.
Molecular typing can be a valuable adjunct to histopathology. More importantly, these molecular subtypes show a distinct genomic profile that provides guidance for targeted agents that should be evaluated in clinical trials targeting different gastric cancer patient populations. the ToGA trial has demonstrated that the targeted agent trastuzumab in combination with chemotherapy significantly improves survival in patients with HER2-positive advanced gastric cancer, an important event in targeted gastric cancer therapy. The classification system developed through molecular typing studies can be applied to new gastric cancer cases. In the future, it is hoped that these results will facilitate the development of clinical trials to explore patient-specific therapies and ultimately improve patient survival.
Reference.:
[1] CA CANCER J CLIN 2021;71:209–249
[2].Nature. 2014 Sep 11;513(7517):202-9.
[3].Gastroenterology,2013,145(3):554-565.
[4].Nat Med, 2015, 21(5): 449-456.
[5].Proc Natl Acad Sci U S A, 2015, 112(4): 1107-1112.
---------This article is intended to provide scientific information to medical professionals only