Current location: Home > NEWS > Industry news
NEWS
PRODUCTS
Low HER-2 Expression Leads to More Accurate Breast Cancer Treatment
News source: Release time:[2023-01-18]
In 2020, there will be about 19.3 million new cases of malignant tumors worldwide, including about 2.3 million new cases (11.7%) of breast cancer in women, making breast cancer the most common malignant tumor [1]. Breast cancer is also the most prevalent malignancy among Chinese women, with approximately 429,105 new cases of breast cancer and 124,002 deaths expected in 2022, and the incidence and mortality of breast cancer will continue to rise [2-3].
Treatment decisions for breast cancer are usually based on traditional histopathological results. Clinically, there are four main subtypes of breast cancer with different prognoses: Luminal A, Luminal B, human epidermal growth factor receptor 2 (HER-2) positive and triple-negative breast cancer (TNBC).
HER-2 is an important driver gene and prognostic indicator for breast cancer, and a major predictor of efficacy of anti-HER-2 drug therapy. In HER-2-positive breast cancers, ERBB2 gene amplification leads to HER-2 overexpression, and patients without anti-HER-2 therapy have more aggressive tumors and worse patient prognosis.Currently, various targeted agents for HER-2 have significantly improved the clinical prognosis of early and advanced HER-2-positive breast cancers. About 45%-55% of breast cancers show low HER-2 expression, i.e. immunohistochemistry (IHC) 1+ or IHC 2+ and in situ hybridization (ISH) no amplification of HER-2 gene.With the established efficacy of antibody-drug conjugate (ADC) in patients with HER-2 low-expressing breast cancer, novel ADCs have become a new treatment option for patients with advanced HER-2 low-expressing breast cancer. [4].
Interpretation of HER-2 low expression
In the Consensus on Clinical Management of Human Epidermal Growth Factor Receptor 2 Low Expression Breast Cancer (2022 Edition), HER-2 low expression is defined as HER-2 IHC 1+ or IHC 2+/ISH-. However, the accuracy of the definition of HER-2 low expression needs to be further clarified, and there are clinical trials currently being conducted to explore this.
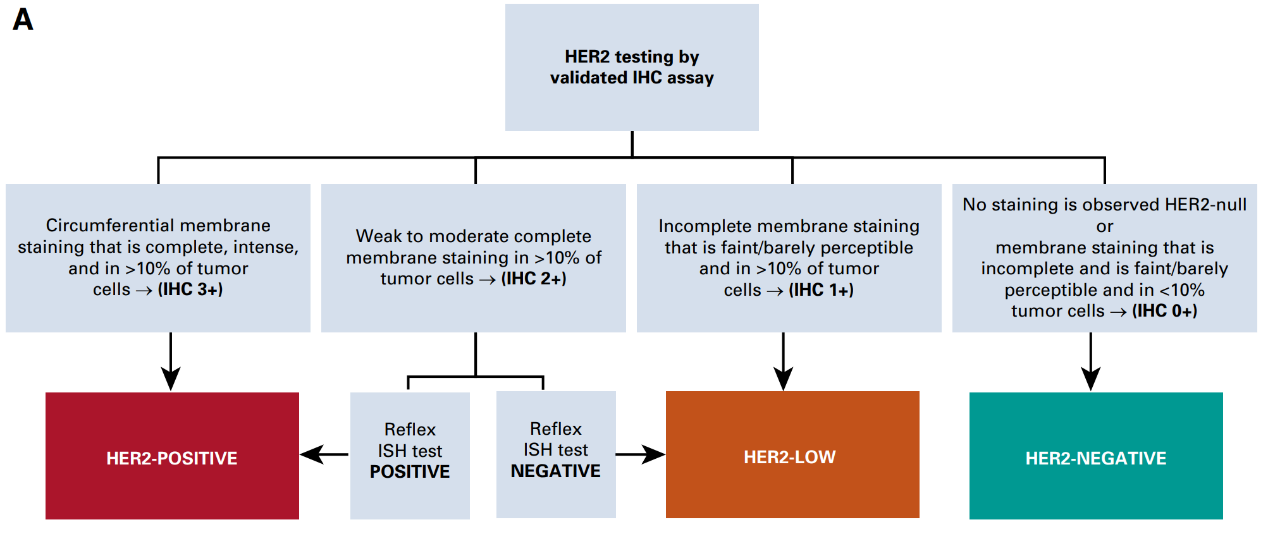
Process for determining low HER-2 expression in breast cancer [5]
T-DXd brings a new landscape of HER-2 treatment for breast cancer
The DESTINY-Breast04 study, the first phase III clinical trial to validate the efficacy of T-DXd in a population with low HER-2 expression, included 557 patients with unresectable and/or metastatic breast cancer with low HER-2 expression who had received prior first- or second-line chemotherapy and were randomly assigned to the T-DXd group or to a chemotherapy group of the physician's choice (e.g. Capecitabine), Eribulin, Gemcitabine, paclitaxel and Albumin-bound paclitaxel). The study results showed that among HR+ patients, median PFS was 10.1 months (95% CI 9.5-11.5) in the T-DXd group compared to 5.4 months (95% CI 4.4-7.1) in the chemotherapy group (HR 0.51; 95% CI 0.40-0.64; p<0.001), with OS of 23.9 months vs 17.5 months (HR 0.64; 95% CI 0.48-0.86; P=0.003) [6].
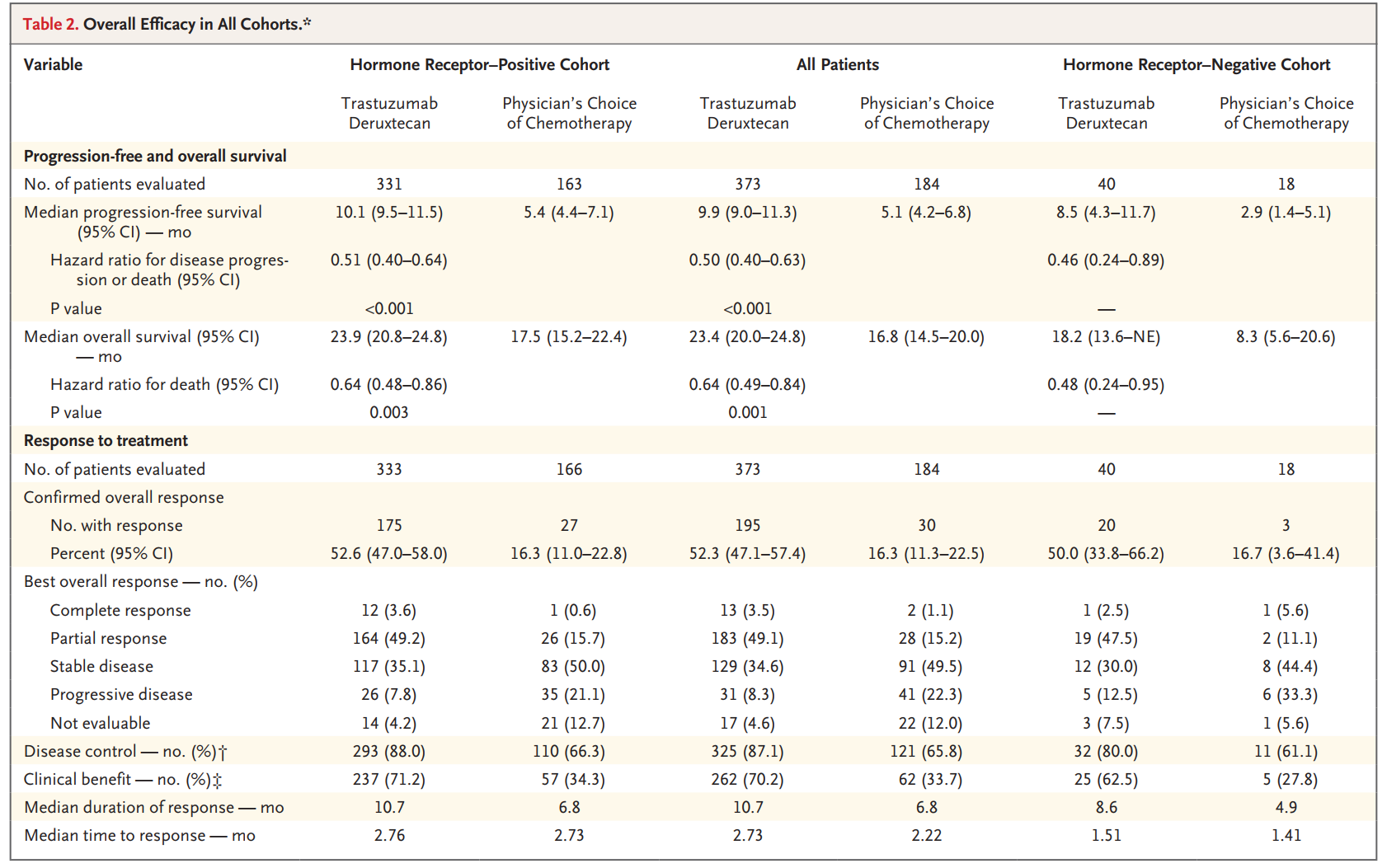
DESTINY-Breast 04 study results
The DESTINY-Breast 04 study's drove a rapid update of the 2022 NCCN guidelines: for patients with unresectable or metastatic breast cancer with low HER-2 expression, who have received at least one previous chemotherapy treatment at the metastatic disease stage and are endocrine refractory if hormone receptor positive (HR+),recommend T-DXd therapy.
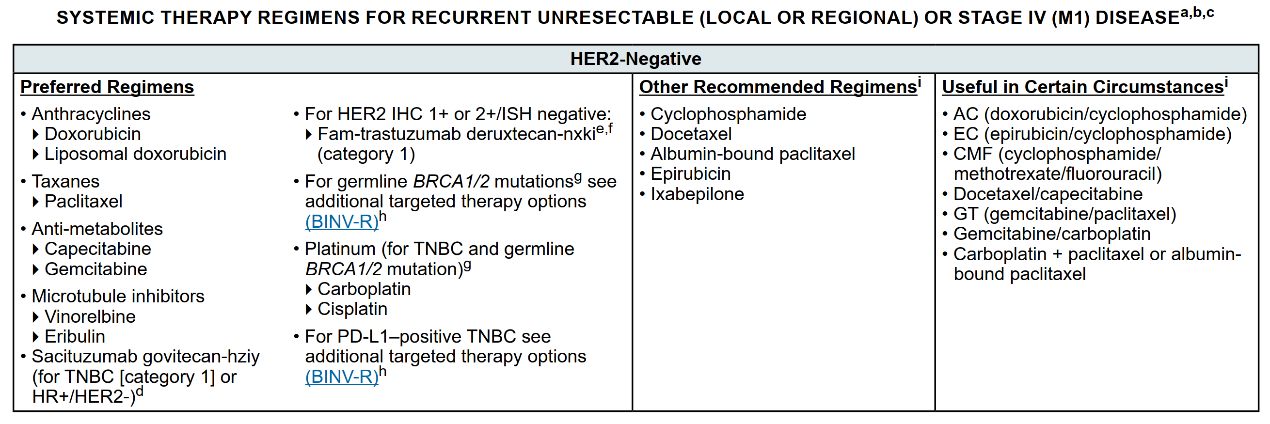
2022.V4 edition of the NCCN breast cancer guidelines [7]
The advent of T-DXd changes the treatment for patients with HER-2 low-expressing breast cancer.
In addition, on August 5, 2022, the U.S. Food and Drug Administration (FDA) approved T-DXd based on DESTINY-Breast 04 for patients with unresectable or metastatic HER-2 low-expressing breast cancer.
This is the first clinical treatment for patients with low HER-2 expression, offering more possibilities for improved survival in breast cancer patients and is of great significance to the field of breast cancer treatment.
Summary
As the data on HER-2 low-expressing breast cancer are still limited at this stage, but clinical trials have shown corresponding benefits, HER-2 low-expressing breast cancer is expected to be a new type of breast cancer treatment, but it is not yet considered a new pathological molecular subtype, and initial treatment of HER-2 low-expressing patients should still be based on molecular typing for treatment options. The current definition of HER-2 low expression as immunohistochemistry (IHC) 2+ in situ hybridisation (ISH)- or IHC 1+ is also being mapped in clinical trials to expand the concept relative to the population, as the expansion of the HER-2 low expression concept means that more patients can benefit from anti-HER-2 therapy and is a further refinement of precision breast cancer treatment.
References
[1]Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries[J]. CA Cancer J Clin, 2021, 71 (3): 209-249.
DOI: 10.3322/caac.21660.
[2]Xia CF, Dong XS, Li H, et al. Cancer statistics in China and United States, 2022: profiles, trends, and determinants[ J]. Chin Med J (Engl) , 2022, 135 ( 5) : 584-590. DOI: 10. 1097/CM9.0000000000002108.
[3]Lei SY, Zheng RS, Zhang SW, et al. Breast cancer incidence and mortality in women in China: temporal trends and projections to 2030[J]. Cancer Biol Med, 2021, 18 (3): 900-909. DOI: 10.20892/j.issn.2095-3941.2020.0523.
[4]China Anti-Cancer Association International Medical and Communication Branch, Breast Cancer Group of Chinese Physicians Association, Oncologists Branch. Consensus on clinical management of human epidermal growth factor receptor 2 low expression breast cancer (2022 edition) [J] . Chinese Journal of Oncology, 2022, 44(12) : 1288-1295. DOI: 10.3760/cma.j.cn112152-20220914-00623.
[5]Tarantino P, Hamilton E, Tolaney SM, Cortes J, Morganti S, Ferraro E, Marra A, Viale G, Trapani D, Cardoso F, Penault-Llorca F, Viale G, Andrè F, Curigliano G. HER2-Low Breast Cancer: Pathological and Clinical Landscape. J Clin Oncol. 2020 Jun 10;38(17):1951-1962. doi: 10.1200/JCO.19.02488. Epub 2020 Apr 24. PMID: 32330069.
[6]Modi S, Jacot W, Yamashita T, et al. Trastuzumab Deruxtecan in Previously Treated HER-2-Low Advanced Breast Cancer. N Engl J Med. 2022 Jul 7;387(1):9-20. doi: 10.1056/NEJMoa2203690.
[7]2022.V4 NCCN Breast Cancer Guidelines