Current location: Home > NEWS > Industry news
NEWS
PRODUCTS
Review the approved treatment of non-small cell lung cancer in 2022 (FDA&NMPA) ——Macromolecular monoclonal antibodies
News source: Release time:[2023-02-01]
NMPA Approval
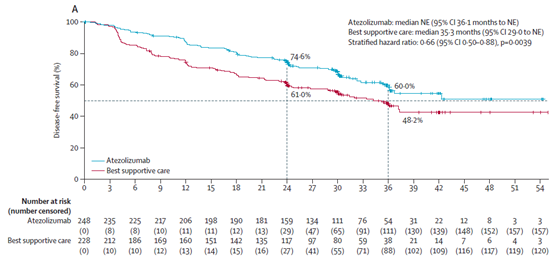
March 18, 2022 Atezolizumab
The National Drug Administration of China (NMPA) has officially approved the company's innovative tumor immune drug Atezolizumab (trade name: Tecentriq) as an adjuvant treatment for patients with stage II-IIIA non-small cell lung cancer (NSCLC) who are evaluated as ≥ 1% tumor cell (TC) PD-L1 positive after surgical resection and platinum-based chemotherapy. The above indications approved by NMPA are mainly based on the research results of IMpower010. IMpower010 is a randomized, open-label, multi-center, phase 3 clinical study in the world, aimed at exploring the efficacy and safety of Atezolizumab and the current best supportive treatment (BSC) for perioperative non-small cell lung cancer patients after surgery and platinum-containing chemotherapy. IMpower010 research results showed that patients with stage II-IIIA NSCLC with PD-L1 ≥ 1% tumor expression, after surgery and chemotherapy, when using Atezolizumab for adjuvant treatment, compared with the current best treatment method, the disease-free survival period (DFS) was prolonged, the risk of disease recurrence or death was reduced by 34% (HR=0.66; 95% CI: 0.50-0.88; p=0.004), and the risk of disease recurrence or death in 3 years was 60% [1].
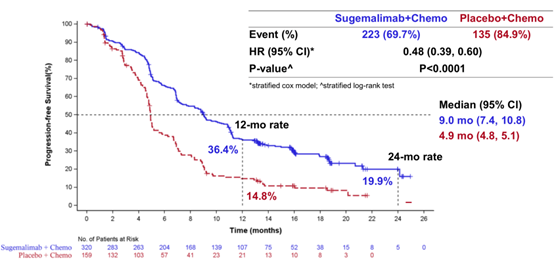
June 2, 2022 Sugemalimab
On June 2, 2022, the drug approval certificate issued by the National Drug Administration of China (NMPA) showed that the application for new indications of the anti-PD-L1 monoclonal antibody Sugemalimab injection from CSTONE PHARMACEUTICALS has been approved in China. According to the press release released earlier by CSTONE PHARMACEUTICALS, Sugemalimab was approved this time to be "used for consolidation treatment of non-resectable stage III non-small cell lung cancer (NSCLC) patients without disease progression after synchronous or sequential radiotherapy and chemotherapy". Previously, the drug has been approved for first-line treatment of specific NSCLC patients in China. GEMSTONE-302 study is a randomized, double-blind, controlled phase III study aimed at evaluating the efficacy and safety of Sugemalimab combined with platinum-containing chemotherapy and placebo combined with platinum-containing chemotherapy in first-line treatment of patients with stage IV NSCLC. The results showed that among all the patients, the median PFS of the Sugemalimab combined chemotherapy group was 9.0 months, which was significantly better than the 4.9 months of the placebo combined chemotherapy group. The median total survival period was extended by 5.1 months, and the risk ratio was HR=0.48 (p<0.0001), which could reduce the risk of disease progression or death by 52% [2].
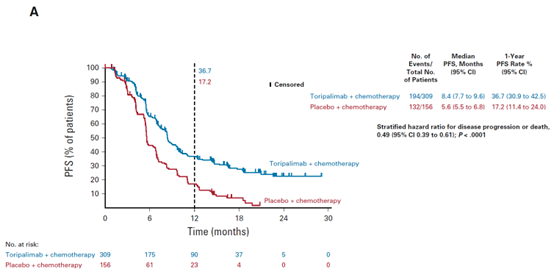
September 19, 2022 Toripalimab
Toripalimab monoclonal antibody ( JS001) is the first approved domestic PD-1 antibody drug. After the domestic launch in 2018, it has been continuously approved for new indications. On September 19, 2022, the new indication of the PD-1 inhibitor Sugemalimab developed by Shanghai Junshi Biosciences Co., Ltd. was approved by NMPA: the combination of Pemetrexeddisodium and platinum was used for the first-line treatment of locally advanced or metastatic non-small cell lung cancer with negative EGFR gene mutation and negative ALK, which could not be resectable. This is the sixth indication approved by Toripalimab, and the first indication in the field of lung cancer treatment. Previously, the drug has been approved for the treatment of melanoma, nasopharyngeal carcinoma, urothelial carcinoma and esophageal squamous cell carcinoma. This approval is mainly based on a multi-center phase III randomized double-blind controlled clinical study (CHOICE-01). The research results have been accepted by the international top academic journal of Clinical Oncology. Compared with the placebo combined chemotherapy group, the PFS of the Toripalimab combined chemotherapy group was significantly prolonged (8.4 months vs 5.6 months), the risk of disease progression was reduced by 51%, HR=0.49 (95% CI: 0.39-0.61), bilateral p<0.0001; The 1-year PFS rate of the Toripalimab combined chemotherapy group was more than twice that of the placebo combined chemotherapy group (36.7% vs 17.2%). Whether squamous cell carcinoma or non-squamous cell carcinoma, PD-L1 expression positive or negative, TMB level high or low, this protocol can bring significant benefits to PFS. In addition, further analysis of WES results showed that patients with FA-PI3K-Akt pathway mutations in non-squamous cell carcinoma and squamous cell carcinoma subgroups could obtain significantly longer PFS and OS in the combination chemotherapy group with Toripalimab; Patients with mutations in IL-7 signaling pathway gene and SWI/SNF pathway gene have significantly better PFS [3].
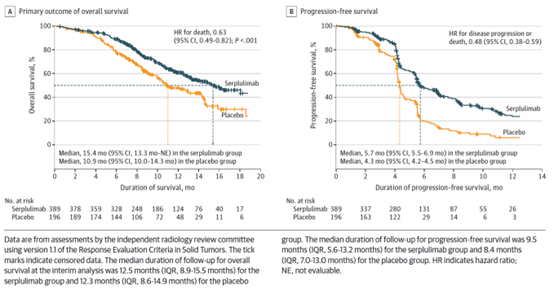
November 1, 2022 Serplulimab
On November 1, 2022, the National Drug Administration of China (NMPA) approved the application for marketing of the innovative PD-1 inhibitor Serplulimab in combination with Carboplatin and albumen-Paclitaxel for first-line treatment of locally advanced or metastatic squamous non-small cell lung cancer (sqNSCLC).The data showed that in the Chinese population, the progression-free survival (PFS) of the first-line treatment of sqNSCLC with Serplulimab combined with chemotherapy reached 9.79 months, and the PFS was extended by more than 4 months, and the objective response rate (ORR) of chemotherapy was increased by 20%.
FDA Approval
March 4, 2022 Opdivo neoadjuvant therapy
On March 4, 2022, the US FDA approved the combination of the PD-1 inhibitor Opdivo (nivolumab) and platinum-containing dual-drug chemotherapy as a new adjuvant therapy for patients with resectable non-small cell lung cancer (NSCLC), regardless of the PD-L1 expression.Opdivo combined chemotherapy is the first immunotherapy combination approved to treat non-small cell lung cancer before surgery. This application is based on the results of the critical phase 3 clinical trial CheckMate-816. In the trial, Opdivo combined chemotherapy showed a statistically significant and clinically significant improvement in pathological complete remission (meaning that there was no sign of cancer in the resected tissues or tissue biopsy samples, pCR) and event-free survival (EFS) when compared with chemotherapy alone before surgery. The safety characteristics of Opdivo plus chemotherapy are consistent with the previously reported NSCLC study. The results released at the AACR conference in April last year showed that the combination of Bristol-Myers Squibb's Opdivo and chemotherapy increased the pathological complete remission rate of patients to 24% when treating resectable non-small cell lung cancer before surgery, compared with 2.2% in the chemotherapy group. [5]。
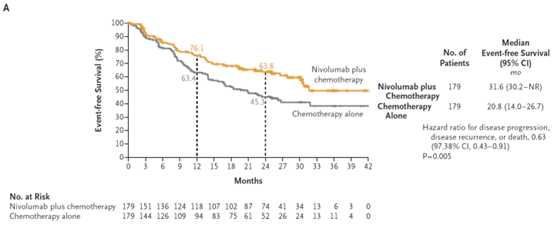
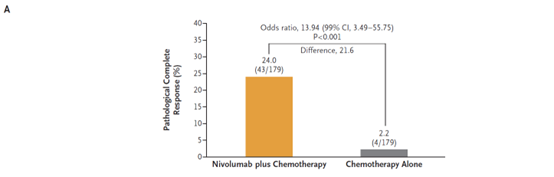
August 1, 2022 Enhertu(DS-8201)
On August 1, 2022, AstraZeneca and Daiichi Sankyo Company Limited announced that the new indication marketing application (sBLA) of the HER2 ADC drug Enhertu (DS-8201) was accepted by the US FDA and granted priority review qualification for patients with unresectable or metastatic HER2 low expression (IHC 1+or IHC2+/ISH negative) breast cancer who had previously received a front line treatment. The accelerated approval of this indication is mainly based on the efficacy data of DESTINY-Lung02 [6]. Among the 52 patients of the main therapeutic group in the DESTINY-Lung0 study, the median age was 58 years (30-78), and women accounted for 69%; Asian Americans accounted for 79%, whites for 12%, and other races for 10%. The primary efficacy end point was the confirmed objective response rate (ORR) and response duration (DOR) according to the RECIST v1.1 standard and the blind independent center evaluation. The confirmed ORR was 58% (95% CI 43, 71), and the median response duration (DOR) was 8.7 months (95% CI 7.1, NE).

November 8, 2022 Cemiplimab Combined platinum chemotherapy
On November 8, 2022, FDA approved the combination of the PD-1 inhibitor Cemiplimab-rwlc (Libtayo, Cemiplimab) and platinum chemotherapy for the first-line treatment of adult patients with advanced non-small cell lung cancer. It is worth mentioning that the approval of this first-line scheme is not limited to PD-L1 expression status. FDA's approval is based on the data of EMPOWER-Lung 3, the global phase 3 trial, which studies the comparison between the platinum dual-drug chemotherapy (Libtayo combination) selected by Libtayo and the single platinum dual-drug chemotherapy. Of the enrolled patients, 43% had squamous tissue tumors, 67% had tumors with PD-L1 expression<50%, 15% had locally advanced diseases that could not be operated, which did not meet the conditions for definitive radiotherapy and chemotherapy, and 7% had pre-treatment and clinically stable brain transfer. Compared with the placebo+ chemotherapy group, the OS of the Cemiplimab-rwlc+ platinum chemotherapy group had statistically significant and clinically significant improvement (risk ratio [HR] was 0.71 [95% CI: 0.53-0.93], bilateral p value=0.0140). The median OS of cemiplimab-rwlc +chemotherapy group and placebo+ chemotherapy group were 21.9 months (95% CI: 15.5, not evaluable) and 13.0 months (95% CI: 11.9, 16.1), respectively [7].
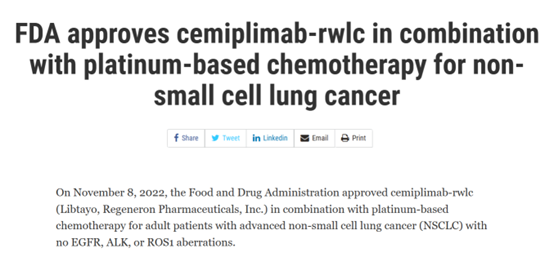
November 10, 2022 PD-L1(durvalumab)+CTLA4(Tremelimumab)
On November 10, 2022, the FDA official website showed that AstraZeneca's anti-CTLA-4 monoclonal antibody, Tremelimumab (Imjudo), was approved as a new indication for first-line treatment of patients with metastatic non-small cell lung cancer (mNSCLC) in combination with durvalumab (Imfinzi) and platinum chemotherapy. These patients do not carry epidermal growth factor receptor (EGFR) mutations or anaplastic lymphoma kinase (ALK) genomic tumor aberrations.
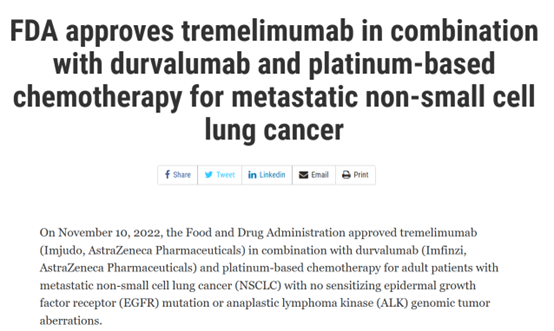
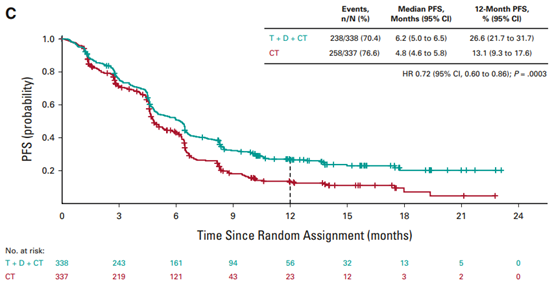
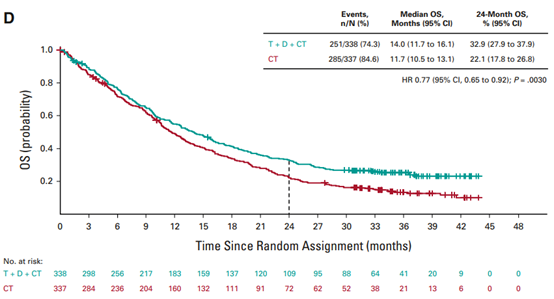
FDA's approval is based on the comparison results of treatment group ① and treatment group ③ in the POSEIDON phase III study [9]. (Treatment group ① Imjudo + Imfinzi+ platinum chemotherapy; treatment group ② Imfinzi+ platinum chemotherapy; treatment group ③ platinum chemotherapy) The results showed that compared with treatment group ③, the OS of patients in treatment group ① improved statistically and clinically (risk ratio: 0.77, 95% CI: [0.65,0.92], bilateral p value=0.00304). The median OS of treatment group ① and treatment group ③ was 14 months (95% CI: 11.7,16.1) and 11.7 months (95% CI: 10.5,13.1) respectively; The median PFS was 6.2 months (95% CI: 5.0,6.5) and 4.8 months (95% CI: 4.6,5.8) respectively (risk ratio: 0.72, 95% CI: [0.60, 0.86], bilateral p value=0.00031).
References &Materials:
[1]Lancet. 2021 Oct 9;398(10308):1344-1357.
[2] Lancet Oncol. 2022 Feb;23(2):220-233.
[3]J Clin Oncol. 2022 Oct 7;JCO2200727.
[4]JAMA. 2022 Sep 27;328(12):1223-1232.
[5]N Engl J Med. 2022 May 26;386(21):1973-1985.
[6] FDA grants accelerated approval to fam-trastuzumab deruxtecan-nxki for HER2-mutant non-small cell lung cancer | FDA
[7] FDA approves cemiplimab-rwlc in combination with platinum-based chemotherapy for non-small cell lung cancer | FDA
[8] FDA approves tremelimumab in combination with durvalumab and platinum-based chemotherapy for metastatic non-small cell lung cancer | FDA
[9] J Clin Oncol. 2022 Nov 3;JCO2200975.