Current location: Home > NEWS > Industry news
NEWS
PRODUCTS
Same Treatment for Different Diseases: How many of the approved "pan-cancer targeted drugs” do you know about?
News source: Release time:[2022-08-08]
01What is pan-cancer?
To enable further analysis of commonalities and differences between diseases in cancer types and organs of origin, the Pan-Cancer program (Pan Cancer) was launched at the TCGA meeting in Santa Cruz, California, October 26-27, 2012.). The goal of Pan-Cancer is to collect coherent and consistent TCGA datasets across tumor types and platforms, then analyze and interpret these data. Pan-Cancer does not refer to a specific tumor. Through pan-cancer analysis, we can find similarities and differences between genomic and cellular changes in different tumor types, and find some general patterns.
02 Clinical Research (NCI-MATCH)
The National Cancer Institute's Molecular Analysis Treatment Selection Program (NCI-MATCH) is the largest-ever precision medicine study hosted by the National Cancer Institute (NCI) to study conditions regardless of the tumor type a patient has. (Location and pathological type of cancer). Treatment of patients with tumors for specific genetic mutations with corresponding drugs is focusing on the increasing number of oncology targets and targeted drugs, and find more effective methods for evaluating drug efficacy in clinical trials.
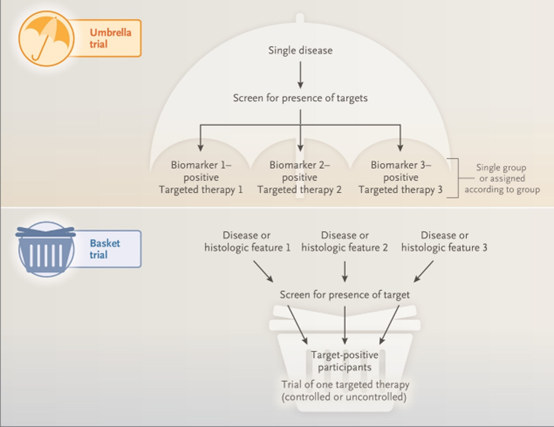
Basket Test - (same disease and same treatment)
The basket trial was first proposed by the American Association for Cancer Research (AACR) in 2014 as an innovative clinical trial for precision cancer medicine. Basket trials refer to targeting multiple tumor types with the same gene mutation as research objects, rather than breaking the framework of tumor location and morphological characteristics based on tumor location and pathological type.
That is to say, as long as a patient has a tumor with a specific gene mutation, it can be considered for inclusion in a clinical trial for specific targeted drug therapy, which is essentially a drug for different tumors.
Umbrella test - (same disease but different treatment)
The Umbrella Trial is a prospective clinical trial evaluating multiple targeted therapies for a single disease stratified into multiple subgroups (based on predictive biomarkers or other predictive patient risk factors).
The umbrella test compares a certain type of disease to an umbrella, such as holding up a big umbrella (such as lung cancer), gathering different lung cancer driver genes, such as KRAS, EGFR, ALK, etc., under the same umbrella. The umbrella is to complete the detection of different targets at the same time, and then allocate different precise targeted drugs according to different target genes.
03 Clinical Application
Approved pan-cancer drug (small molecule inhibitor)
1)Larotrectinib
On November 26, 2018, Bayer announced that the US FDA approved the targeted drug larotrectinib (trade name: Vitrakvi) for the treatment of patients carrying NTRK gene fusions with unknown acquired resistance mutations, metastasis or surgical resection may lead to severe complications, adult and pediatric patients with pan-solid tumors without satisfactory alternative treatment options or who have failed prior therapy. This is the first non-tumor/tissue-related targeted drug approved by the US FDA, which caused a global sensation for a while.
On April 13, 2022, Bayer announced that China's NMPA approved the launch of the targeted drug larotrectinib sulfate capsules (trade name: Vitech) for the treatment for patients with NTRK gene fusions , with unknown acquired resistance mutations, locally advanced ,metastasis or surgical resection may lead to severe complications, adult and pediatric patients with pan-solid tumors without satisfactory alternative treatment options or who have failed prior therapy.
2)Entrectinib
2019, August 15, 2019 FDA accelerated the approval of Entratinib (ROZLYTREK) for adult and pediatric solid tumor patients for age of 12 and older with Neurotrophic Tyrosine Receptor Kinase (NTRK) gene fusions without known acquired resistance mutations, who are metastatic or surgical resection which may result in serious complications, progression after treatment or without satisfactory standard of care. In addition, the FDA approved entrectinib for adults with metastatic non-small cell lung cancer (NSCLC) with tumors of ROS1-positive.

▲(Source: FDA official website)
Dabrafenib)+ Trametinib)
On June 22, 2022, the FDA approved the BRAF/MEK inhibitor combination therapy of dabrafenib and trametinib for the treatment of 6 patients who have progressed after prior therapy and have no satisfactory alternative treatment options. Adult and Pediatric Patients Aged and Over with Unresectable or Metastatic Solid Tumors with BRAF V600E Mutation. The combination regimen of dabrafenib + trametinib is not suitable for colorectal cancer patients due to known intrinsic resistance to BRAF inhibition. Dabrafenib is not indicated for patients with wild-type BRAF solid tumors.

▲(Source: FDA official website)
Approved pan-cancer drug (PD-1/PD-L1 monoclonal antibody)
1)Pembrolizumab
On May 23, 2017, the FDA granted accelerated approval to pembrolizumab for the treatment of patients with unresectable or metastatic dMMR/MSI-H advanced solid tumors, regardless of tumor site or histology. The drug is the first FDA-approved PD-1 drug. In the KEYNOTE-158 study, the ORR of 49 patients of recurrent endometrial cancer with high MSI was 57%, of which 16% achieved CR and 41% achieved PR. These results suggest that all patients with recurrent endometrial cancer should be assessed for MSI status.
▲(Source: FDA official website)
2)Pembrolizumab
On June 16, 2020, the U.S. Food and Drug Administration (FDA) granted accelerated approval to pembrolizumab (KEYTRUDA) for the treatment of adult and pediatric patients with unresectable or metastatic tumors with high mutational burden (TMB-H) [≥ 10 mutated/megabase (mut/Mb)] solid tumor, FDA-approved detection reagent, that has progressed after prior therapy and for which there is no satisfactory alternative treatment option. In addition, the FDA approved the Foundation One CDx test (Foundation Medicine, Inc.) as a companion diagnostic for pembrolizumab.

▲(Source: FDA official website)
3)Dostarlimab-gxly
On August 17, 2021, the FDA granted accelerated approval to the expanded indication of dostarlimab for the treatment of patients with mismatch repair deficiency (dMMR) who have progressed during or after prior therapy and have no satisfactory alternative treatment options for relapse or advanced disease adult patients with solid tumors.

▲(Source: FDA official website)
Challenges and thoughts on pan-tumor target therapy?
In different tumor types, it cannot be said that if the driver gene is found, the corresponding targeted drugs can be used. It is not simple to treat different diseases with the same treatment. So, why do different tumor types have the same mutation, and there will be differences in efficacy? Welcome to leave a message for discussion.
This article is only used to provide scientific information to medical professionals and does not represent the views of this platform.