Current location: Home > NEWS > Industry news
NEWS
PRODUCTS
FH-deficient uterine leiomyoma and renal cell carcinoma
News source: Release time:[2024-07-17]
Foreword
Uterine leiomyomas are benign mesenchymal cell tumors of smooth muscle origin, with various morphologies, and are the most common benign tumors in women. The prevalence rate in women of childbearing age is up to 25% and that in perimenopausal women is up to 70%. Most uterine fibroids have no obvious symptoms, and about 25% of them have severe symptoms and need treatment [1–2]. Fumarate hydratase (FH) deficient uterine leiomyomas are a rare type of uterine leiomyomas that have more severe symptoms and a poorer prognosis than other subtypes of uterine leiomyomas.
FH-deficient renal cell carcinoma (FHRCC) has an early onset age, atypical imaging findings, and variable pathological morphology, making clinical diagnosis extremely difficult. It is highly invasive and prone to early distant metastasis such as lymph node metastasis and bone metastasis, and lacks of effective and systematic treatment [3]. Gene detection plays an important role in the diagnosis and treatment of FH-RCC.
Molecular mechanisms of uterine leiomyoma
Typical leiomyomas account for about 90% of all leiomyomas, and the remaining 11 subtypes together account for about 10%[4]. Uterine borderline leiomyomas include diffuse leiomyomatosis (DUL), intravenous leiomyomatosis (IVL), metastatic leiomyomatosis (BML), and STUMP. The incidence of preexisting malignant lesions in leiomyomas is generally considered to be < 0.5% (0.13%–2.02%) [2]. Most leiomyomas arise from mutations in a single stem cell; Multiple coexisting tumors may have the same clonal origin and different subclone gene variations [4].
Whole genome sequencing (WGS) suggests that uterine leiomyomas develop from normal uterine muscle cells through at least four different genetic aberrations. Most lesions are caused by MED12 mutations, and the less common pathway is FH biallelic inactivation, which is accompanied by chromosome fragmentation and gene rearrangement [5].
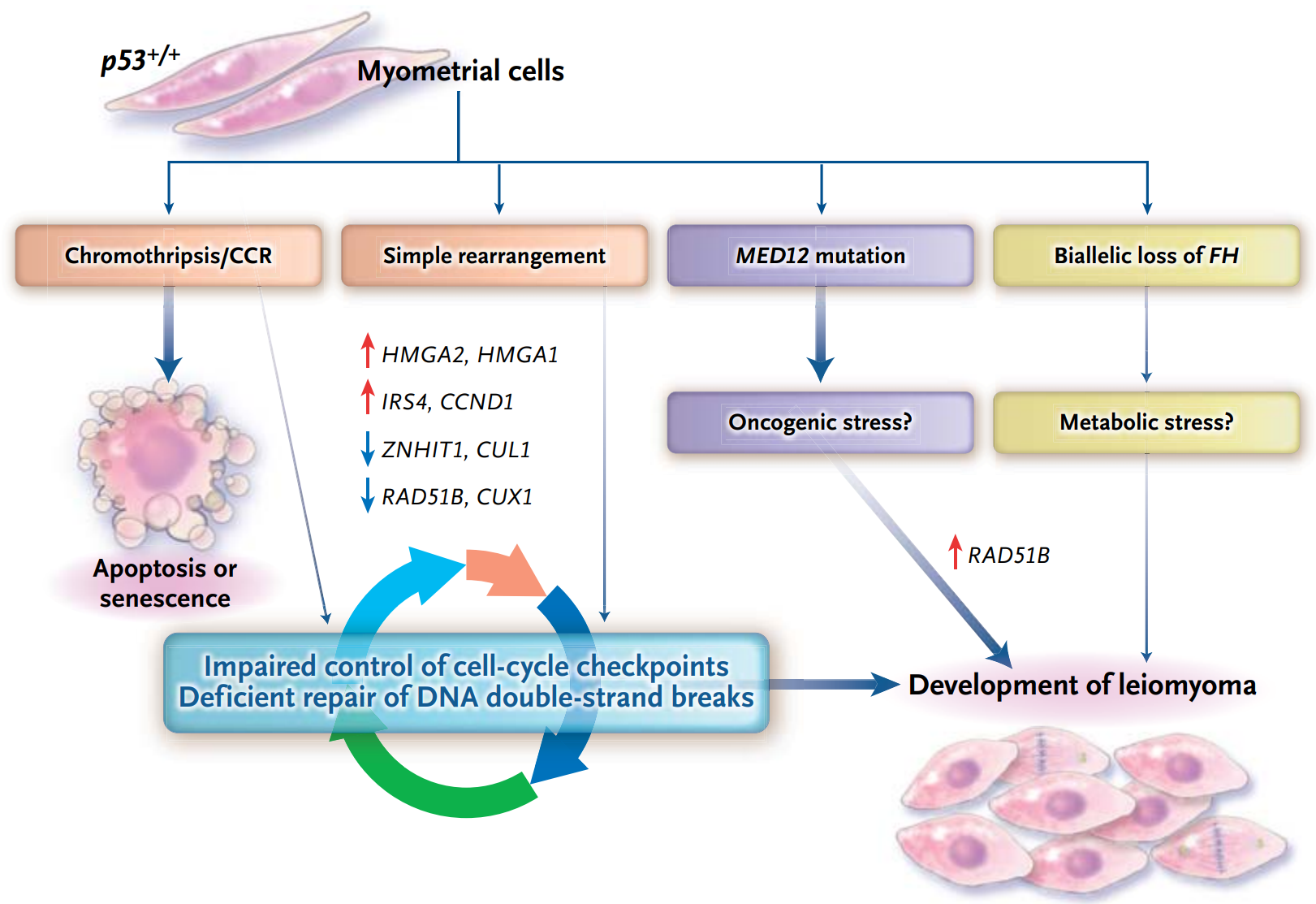 Molecular mechanisms of uterine leiomyomas [5]
Molecular mechanisms of uterine leiomyomas [5]
MED12 gene mutations occur in more than 70% of patients with uterine fibroids [4], most of which occur in exon 2, and the missense mutation at codon 44 accounts for more than half. Other common mutations include exon 1 mutation, intron 1-exon 2 intraframe insertion loss (indel), L1224F, and deletion [6]. MED12 gene mutation is an important marker of sporadic uterine fibroids. The frequency of HMGA1/2 gene fusion in uterine fibroids is 25%–29%, and the main fusion form is hmga2-RAD51B[4]. These tumors are easily detected by HMGA2 immunohistochemistry. FH gene mutation only occurs in about 1% of uterine fibroids, but this part of the patient may be associated with hereditary syndrome. The above gene variations are usually mutually exclusive [4].
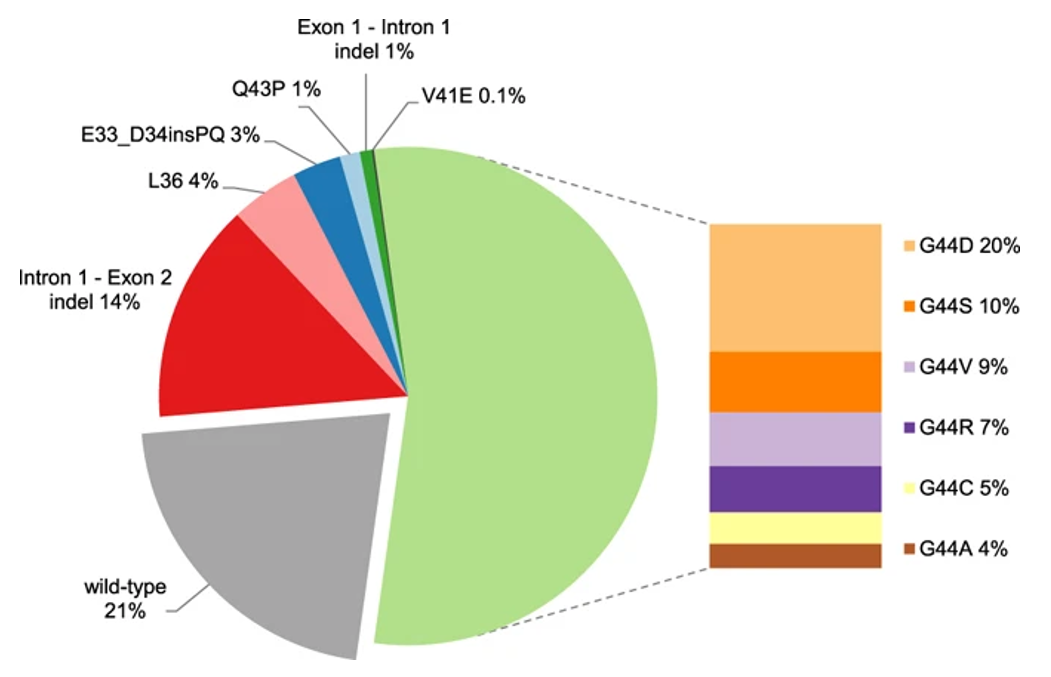 The main mutations and frequency of MED12 gene [6]
The main mutations and frequency of MED12 gene [6]
Hereditary leiomyomatosis and renal cell carcinoma syndrome
FH-deficient uterine leiomyoma is a rare type of uterine leiomyoma, accounting for 0.4%–1.6% of all uterine leiomyomas, and is associated with embryogenic or systemic mutations of the FH gene. Hereditary leiomyomatosis and renal cell carcinoma (HLRCC) syndrome are autosomal dominant genetic diseases caused by embryogenic mutation of FH gene, presenting as multiple leiomyomas of the skin, early-onset uterine leiomyoma and renal cell carcinoma [7].
Skin lesions are the most sensitive and specific clinical manifestation of HLRCC. Skin lesions in HLRCC syndrome are characterized by multiple skin or hair leiomyomas with stabbing pain. When uterine leiomyoma occurs as a concomitant disease of HLRCC syndrome, it is generally manifested as the large number and volume of leiomyomas. Patients generally have a younger onset age, which is 6–12 years earlier than that of common uterine fibroids. The recurrence rate after myomectomy is higher. The lifetime risk of renal cancer in HLRCC is approximately 15%, which is the leading cause of death for our patient. The histological type is mainly type II papillary renal cell carcinoma (pRCC), which is mostly unilateral and often comes to an advanced stage at the presentation with a very poor prognosis [7].
Clinical diagnostic criteria of HLRCC [7]
Main criteria | Multiple cutaneous leiomyomas, especially with characteristic stabbing pain |
One or more hair leiomyomas with characteristic stabbing pain | |
Secondary criteria | Solitary skin leiomyoma and HLRCC family history |
PRCC Form II before age 40 | |
Women develop uterine fibroids with severe symptoms before the age of 40 | |
A first-degree relative who meets one of the above conditions and a female member of a second-degree paternal family who, before the age of 40, has uterine fibroids with severe symptoms |
Because of its early characterization, easy detection and good prognosis, uterine leiomyomas can serve as benign sentinel tumors for HLRCC-related renal cancer. Myomectomy is the first choice for infertile patients, and it is recommended to complete the birth as soon as possible. hysterectomy is recommended for those without fertility demand, and close follow-up is required after operation. In order to intervene as early as possible and improve the prognosis, clinical screening of HLRCCs associated with HLRCCs should be emphasized [7].
A Phase II clinical trial (NCT01130519) included 83 patients with pRCC, including 42 patients with HLRCC and 41 patients with sporadic pRCC, treated with bevacizumab in combination with erlotinib. The primary endpoint was the objective response rate (ORR), and the secondary endpoints were progression-free survival (PFS) and duration of response (DOR). The ORR was 51% in all patients, 72% in patients with HLRCC and 35% in patients with sporadic pRCC. The mPFS were 14.2 months in all patients, 21.1 months in patients with HLRCC, 8.9 months in patients with sporadic pRCC, and 44.6 and 18.2 months in median OS [8–9]. This indication has not been approved by the FDA, and the NCCN guidelines recommend bevacizumab plus erlotinib for patients with advanced pRCC including HLRCC (category 2A recommendation) [10].
Detection method of FH defect
FH gene encodes fumarate hydratase, which catalyzes the reversible hydration and dehydration reactions between fumarate and malate in mitochondria, playing a key role in the tricarboxylic acid (TCA) cycle [7]. FH-deficient cells lose the ability to metabolize fumaric acid, and the intracellular fumaric acid level increases, resulting in reduced mitochondrial oxidative phosphorylation, promotion of aerobic glycolysis, induction of pseudohypoxia, post-translational protein modification and homologous recombination to repair the defects, leading to the occurrence of tumor [11]. When the patients meet the corresponding clinical diagnostic criteria, it indicates that the probability of HLRCC syndrome is very high, and further tests, such as immunohistochemistry (IHC) analysis and gene detection, are needed to assist in the diagnosis [7].
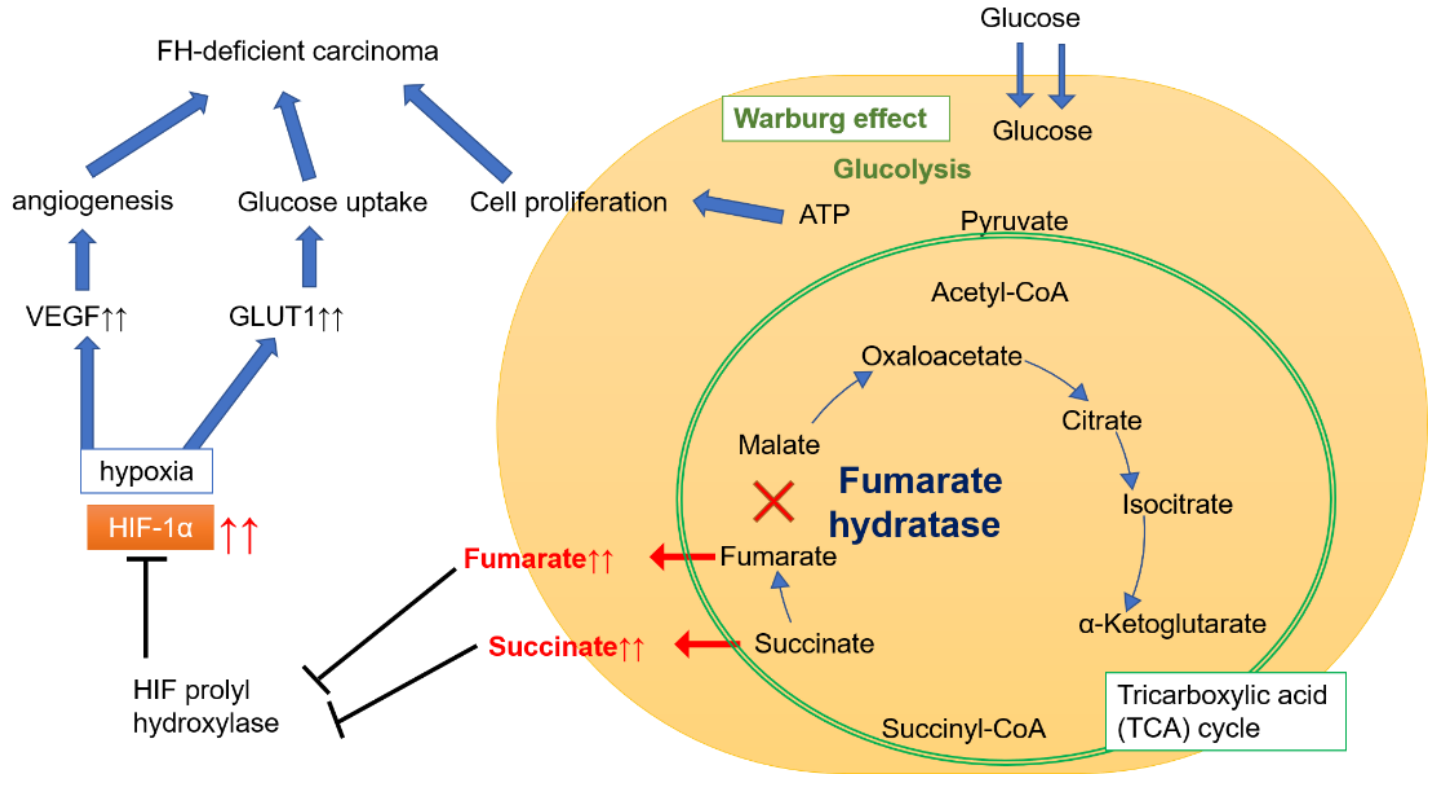
Energy metabolism pathway of FH defect tumor [12]
The deletion of FH IHC staining is highly specific and is closely related to FH gene mutations (systemic or embryogenic mutations). However, FH positive staining should not rule out FH gene abnormalities. FH missense mutations can produce non-functional FH proteins that limit the specificity of IHC staining. FH-dependent IHC alone may cause missed diagnosis in some patients, and cysteine 2- succinate (2SC) and AKR1B10 staining can be used as a complement to FH IHC [3,7]. At present, gene detection is still the gold standard for the diagnosis of FH-deficient uterine leiomyoma [7].
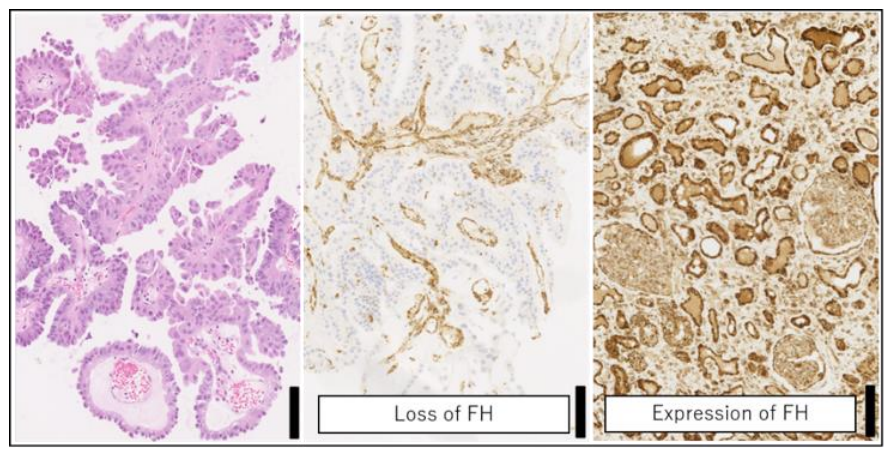 Morphology of renal biopsies from patients with HLRCC and FH immunohistochemistry [12]
Morphology of renal biopsies from patients with HLRCC and FH immunohistochemistry [12]
FH gene is located in chromosome 1q42.3–43, and has a total length of about 22 kb and consists of 10 exons [11]. The molecular genetic mechanism of FH mutation may include loss of heterozygosity (LOH), epigenetics, and copy number variation (CNV). The LOH of 1q43 is very common in patients with HLRCC (80%), suggesting biallelic inactivation of the FH gene, with one allele inactivated by embryogenic mutation and the other inactivated by LOH [7]. FH mutations have a high degree of randomness, and there is no clear hot spot for high-frequency mutations. More than 280 pathogenic and potentially pathogenic FH mutations associated with HLRCC have been identified, with the most common mutation type being missense (50%–60%), followed by nonsense and frameshift mutations (approximately 30%). 3%–5% of patients have FH large fragment deletion [3].
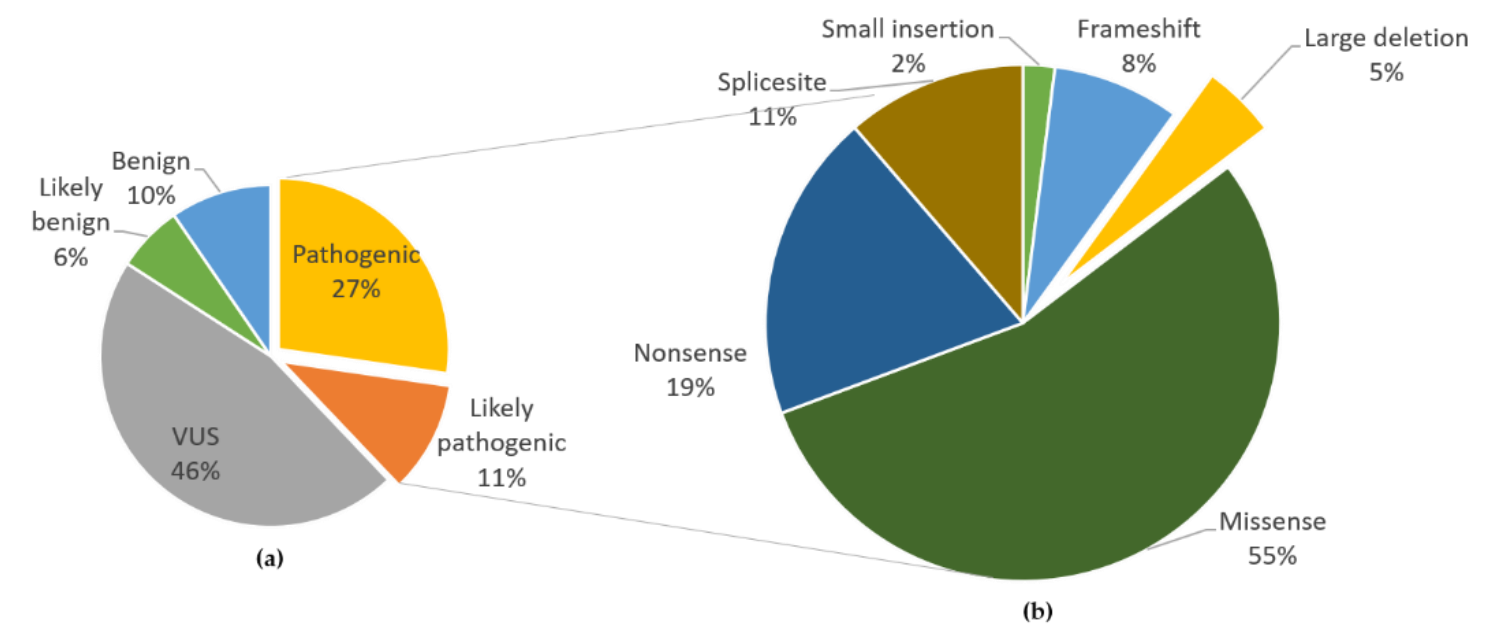 The main mutation form and frequency of FH gene [12]
The main mutation form and frequency of FH gene [12]
Some FH-deficient uterine leiomyomas are caused by systemic mutation of FH gene. Studies have shown that systemic mutations of FH gene are more common than blastocyst mutations (the ratio of blastocyst to blastocyst in Feishuo data is about 2:3). 60%–80% of patients with FH-RCC have embryogenic FH mutations. Embryogenic and systemic FH mutations in patients with suspected FHRCC need to be detected simultaneously [3].
Summary
In summary, comprehensive genetic testing is essential for the diagnosis and treatment of FH-deficient uterine leiomyoma and renal cell carcinoma. The FH/MED12 mutation assay in Fisher Biology qualitatively detects FH fully encoded regions (CDS) and MED12 hot spots in tissue and/or blood samples. The indel, CNV and other mutations of FH gene can be detected to assist in the diagnosis of HLRCC, indicating the efficacy of targeted therapy in patients with FH-deficient RCC.
References
[1] China expert consensus on the diagnosis and treatment of uterine fibroids
[2] BJOG. 2017 Sep; 124(10):1501-1512.
[3] Consensus on clinical diagnosis and treatment of fumarate hydratase deficiency renal cell carcinoma
[4] fifth edition WHO classification of female reproductive system tumors
[5] N Engl J Med. 2013 Jul 4; 369(1):43-53.
[6] Fertil Steril. 2014 Oct; 102(4):1137-42.
[7] China expert consensus on the diagnosis and treatment of fumarate hydratase deficiency uterine leiomyoma (2023 edition)
[8] Journal of Clinical Oncology 38, no. 15_suppl (May 20, 2020) 5004-5004.
[9] ClinicalTrails database
[10] NCCN guidelines for diagnosis and treatment of renal cancer 2024 v1
[11] J Clin Pathol. 2021 Oct; 74(10):615-619.
[12] Int J Mol Sci. 2021 Jul 26; 22(15):7962.
Statement: This article is only for sharing, if it involves copyright issues, please contact us as soon as possible, we will correct the first time, thank you!