Current location: Home > NEWS > Industry news
NEWS
PRODUCTS
Re-exploration of Rare Variation of EGFR to Boost Clinical Accurate Medication
News source: Release time:[2024-06-14]
Lung cancer remains the most common cause of cancer death worldwide, with NSCLC accounting for 80–85%. EGFR (Epidermal Growth Factor Receptor) is a trans-membrane tyrosine kinase receptor that is located on the cell membrane and is an important member of the tyrosine kinase receptor family. Activation of EGFR can promote the growth and differentiation of many cells, including embryonic development, maintenance and repair of adult tissues, and proliferation and metastasis of cancer cells. According to statistics, about 73.9% of patients with NSCLC have driver gene mutations. Among them, about 45.9% of patients with non-small cell lung cancer in China have EGFR mutation, which indicates that about half of patients with non-small cell lung cancer in China can achieve survival benefits with the help of EGFR targeting drugs. Currently, EGFR-targeted drugs have been approved for the treatment of patients with "classical" mutations and a few other mutations. Typically, patients with an EGFR L858R mutation or deletion of exon 19 (19del) are susceptible to first-,second-,or third-generation tyrosine kinase inhibitors (TKIs). Patients with nonclassical mutations of EGFR, however, have variable responses and reduced susceptibility to EGFR inhibitors, including the third-generation inhibitor Osimertinib.
Although targeted therapies have been approved for common EGFR mutations and a small number of other mutations, there are still some EGFR mutations that do not have an effective treatment, and the incidence and drug sensitivity of these mutations are unclear. In 2021, a team from MD Anderson Cancer Center in Houston, Texas, USA published a study in the top-level journal Nature. The study classified EGFR mutations according to the structure and function of EGFR receptor based on the analysis of preclinical model, protein 3D structure and patient response data, and finally identified four subgroups of mutations: classical-like mutation, T790M-like mutation, insertion mutation in the αC- helix C-terminal ring of EGFR exon 20, and mutation (PACC) located on the inner surface of ATP binding pocket or at the end of the α C helix region. Many unusual mutations fall into the class of PACC mutations. This classification is also known as the The University of Texas MD Anderson Cancer Center (MDACC) system.
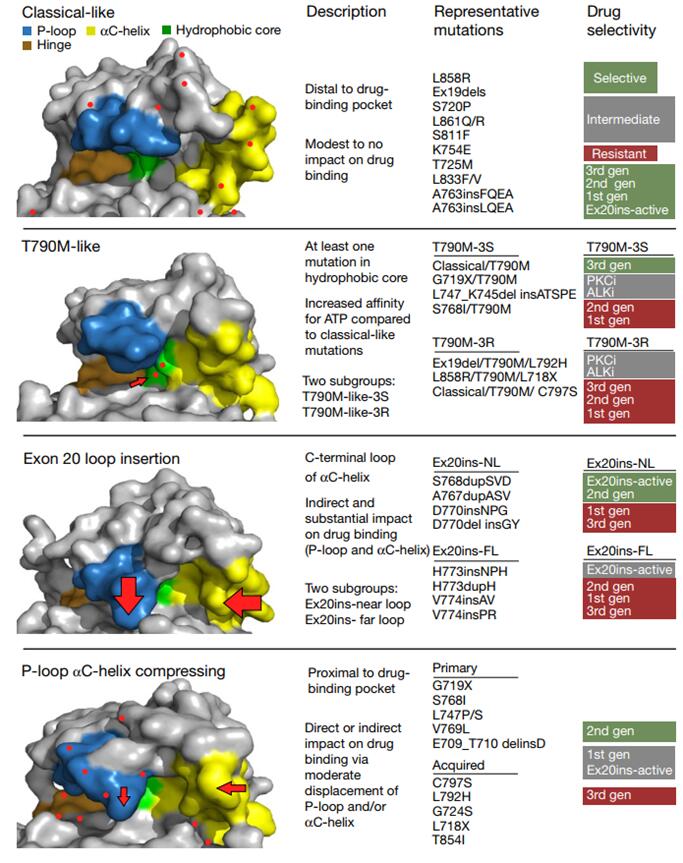
Models for different classifications and drug susceptibility
The study analyzed the molecular biology of 16,715 patients with EGFR-mutated NSCLC from five patient databases with genomic characteristics. Of these, 67.1% had classical mutations in EGFR (L858R and/or 19del with or without T790M); Non-classical EGFR mutations were present in 30.8% of subjects, including EX-20ins mutations (9.1%), other atypical mutations (12.6%), or complex mutations containing non-classical mutations (9.1%). 2.2% had both classical, T790M and nonclassical mutations. Nonclassical mutations of EGFR occurred predominantly in exon 18 (23.7%) and exon 20 (20.9% insertions and 19.2% point mutations). The high incidence of nonclassical mutations was in P-loop(L718-V726,13.6%) and α c-helix of the c-terminal loop (A767-G779, 29.4%).
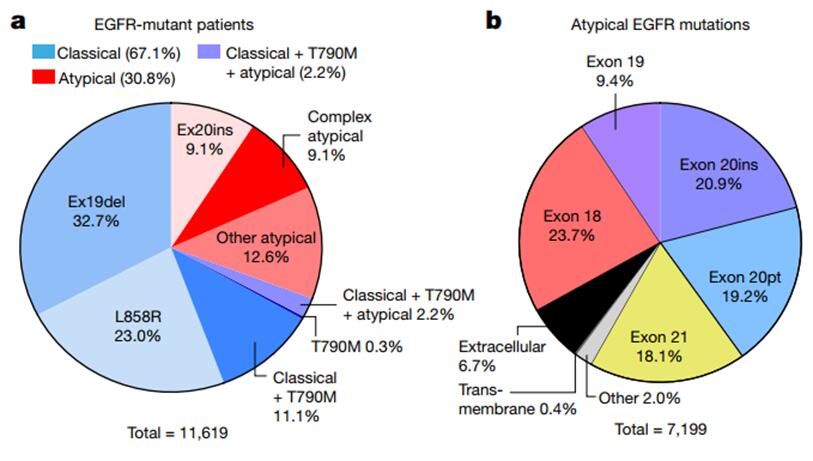
EGFR mutation related features
The investigators established 76 cell lines with EGFR mutations (exons 18–21 of EGFR) and screened these cell lines with 18 generations of EGFR 18-21 TKIs, including ALK inhibitors and PKC inhibitors, to observe and summarize the NSCLC subsets with four EGFR mutations. Comparisons of the associations of different subsets with drug susceptibility to exons revealed that the structure-based subsets were more predictive than the exon-based subsets.
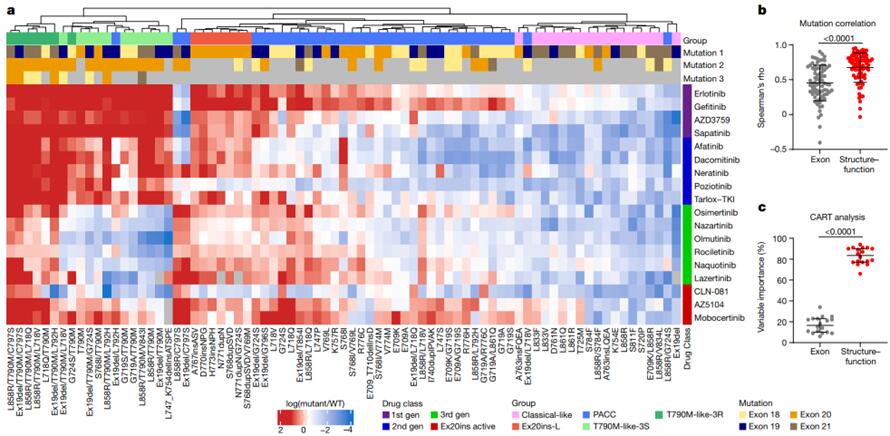
Prediction of Mutations and Comparison of Drug Susceptibilities Based on Structural-Functional Classification and Exon Classification
This study focused on the mutation of PACC subtype and the efficacy of targeted drugs. Mutations in PACC that span exons 18-21 of EGFR, including G719X, L747X, S768I, L792X, and T854I, are expected to alter the orientation of the P- ring or αC- helix. Computer analysis and simulation of the interaction of Osimertinib with the PACC mutations G719S and L718Q predicted that the change in the P-ring direction would change the position of the TKI stability point and cause the indole ring of Osimertinib to deviate from the P-ring, thereby undermining the stability of drug binding. In contrast, second-generation TKIs do not interact with the P- ring of EGFR and maintain points of interaction in hydrophobic fissures. The second-generation TKIs were also found to be significantly more selective for PACC mutations than any other TKIs. Both third-generation TKIs and 20ins active inhibitors developed resistance to the PACC mutation -C797S mutation without the T790M mutation, but the first/second generation TKIs remained susceptible.

Efficacy of PACC subtypes and different generations of EGFR TKIs
In another retrospective study, of the 193 articles, 157 were excluded and 38 were included in the final analysis. Ultimately, a total of 1,838 patients with rare mutations who received TKI were included in the analysis. G719X, L861Q, S768I, E709X, E709-T710delinsD, and L747X were the most common infrequent mutations, with a total of 1095 patients.
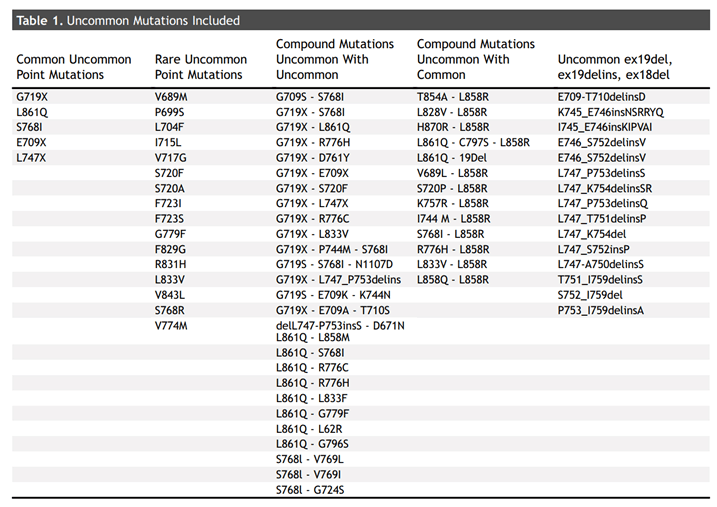
Relevant variants included in the literature
The MDACC system classifies EGFR mutations in the kinase domain by structural function. Researchers categorized unusual EGFR mutations according to the MDACC system and evaluated the clinical response of different generations of TKIs. Similar to the results of other studies, the majority of the rare mutations included in the investigator's analysis corresponded to either PACC mutations or classical-like mutations according to classification. Gene mutations in 621 patients could not be classified according to the MDACC system. For classic-like mutations, the response rates (RRs) for first-,second-and third-generation TKIs were 35.4% (95% CI: 27.2% to 44.2%), 51.9% (95% CI: 44.4% to 59.3%), and 67.9% (95% CI: 47.6% to 84.1%), respectively. For PACC mutations, the RRs for first-,second-and third-generation TKIs were 37.2% (95%CI: 32.4%-42.1%), 59.6% (95%CI: 54.8%-64.3%), and 46.3% (95%CI: 32.6%-60.4%), respectively.
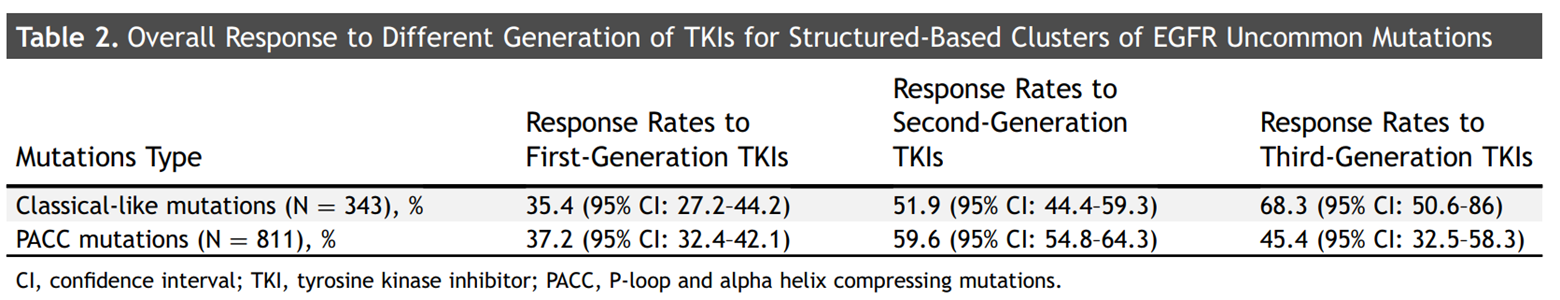
Response of different mutant subtypes of EGFR to TKI drugs
Summary
The mutant types of EGFR in NSCLC have complex loci and strong heterogeneity, and EGFR-TKI has great differences in clinical efficacy. When categorised according to a structured MDACC system, patients who categorise EGFR mutations as PACC mutations have a higher response rate to second-generation TKIs, which also suggests that EGFR-positive patients with NSCLC also need to consider multiple factors further when choosing EGFR TKIs in order to give patients a higher quality of life.
Overall, these two studies provide a more accurate targeted drug selection and prognosis method for patients with NSCLC with EGFR mutation. However, they are both retrospective studies, and further prospective studies are needed to confirm the relevant evidence.
Clinical evidence for treatment options in patients with rare mutations of EGFR is still evolving. The NCCN guidelines have recommended the rare variation of EGFR independently, although there is currently no domestic consensus on the guidelines to recommend it. With the increasing sensitivity of new sequencing technologies and the global adoption of second-generation sequencing, the detection rate of rare EGFR mutations will increase correspondingly. Therefore, more prospective trials need to be conducted against this population, and there will be more clinical evidence to prove that EGFR mutations need to be further distinguished and refined to better guide clinical treatment.
SPACEGEN is committed to providing an overall solution for accurate and individualized diagnosis of tumors, including areas such as early screening, disease diagnosis, individualized medication guidance and efficacy monitoring. At present, SPACEGEN Oncology Multi-Gene Mutations Detection Kit has obtained the Class III medical device registration certificate and CE-IVD certification, which can facilitate the accurate classification of EGFR variations and assist in accurate clinical treatment and individualized medication.
Detection of genes and loci
Gene | Nucleic acid type | Mutation type | Cover exon or fusion partner | |
1 | EGFR | DNA | Gene mutation | 18,19,20,21 |
2 | KRAS | 2,3,4 | ||
3 | BRAF | 15 | ||
4 | PIK3CA | 10,14,21 | ||
5 | NRAS | 2,3 | ||
6 | HER2 | 19,20,21 | ||
7 | MET | 2,14,16,19 | ||
8 | AKT1 | 2 | ||
9 | KIT | 9,11,13,17 | ||
10 | PDGFRA | 12,18 | ||
11 | ALK | RNA | Fusion mutation | EML4-ALK(21 species) |
12 | RET | KIF5B、CCDC6、NCOA4、PCM1、 GOLGA5、HOOK3、KTN1、PRKAR1A(15 species) | ||
13 | ROS1 | CD74、GOPC、SDC4、SLC34A2、EZR、LRIG3、TPM3(15species) | ||
14 | MET | Exon skip | Exon 14 skip |
References:
[1] Robichaux, J.P., Le, X., Vijayan, R.S.K. et al. Structure-based classification predicts drug response in EGFR-mutant NSCLC. Nature 597, 732–737 (2021). https://doi.org/10.1038/s41586-021-03898-1
[2] Borgeaud M, Parikh K, Banna GL, Kim F, Olivier T, Le X, Addeo A. Unveiling the Landscape of Uncommon EGFR Mutations in NSCLC-A Systematic Review. J Thorac Oncol. 2024 Mar 16:S1556-0864(24)00123-0. doi: 10.1016/j.jtho.2024.03.016. Epub ahead of print. PMID: 38499147.