Current location: Home > NEWS > Industry news
NEWS
PRODUCTS
Overview on Immunotherapy Biomarkers
News source: Release time:[2024-05-30]
In recent years, immune checkpoint inhibitors (ICIs), represented by PD-1/PD-L1 inhibitors, have achieved groundbreaking progress in the immunotherapy of various tumors, bringing significant survival benefits to patients. However, not all patients can benefit from immunotherapy. Clinically, it is often necessary to screen suitable candidates and predict the efficacy of immunotherapy through specific biomarkers.
1. Tumor Microenvironment (TME)
1.1 Significance of TME
The tumor microenvironment (TME) refers to the cells and physicochemical components surrounding tumor cells, including adaptive immune cells, myeloid immune cells, immune cells at the intersection of innate and adaptive immunity, tumor stromal cells, stromal components, and vascular cells. The TME comprises both cellular and non-cellular components, such as:- Adaptive immune cells: CD8+ T cells, CD4+ T cells, Tregs, B cells; - Myeloid immune cells: macrophages, neutrophils, monocytes, dendritic cells, mast cells, eosinophils, myeloid-derived suppressor cells (MDSCs), platelets; - Innate and adaptive intersection immune cells: NK cells, iNKT cells, γδT cells, innate lymphoid cells (ILCs); - Tumor stromal cells and components: cancer-associated fibroblasts (CAFs), adipocytes, extracellular matrix (ECM), neurons, and nerve fibers; - Vascular cells: endothelial cells (ECs), lymphatic endothelial cells (LECs), pericytes. Most immune cells exhibit dual roles in both promoting and inhibiting tumor growth [1-2]. The immune microenvironment is one of the ten hallmarks of cancer, and its diversity and complexity significantly impact immunotherapy. Currently, multiple immunotherapy biomarkers have been found to be related to the tumor microenvironment.
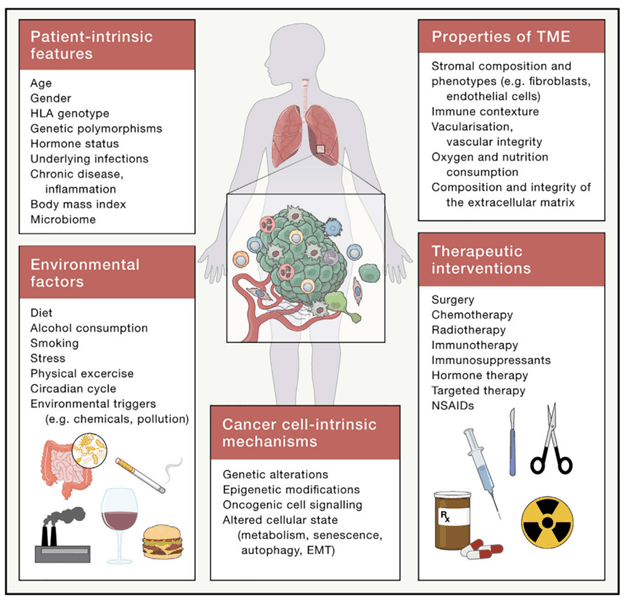
Multiple cellular endogenous and systemic factors influence patients and the TME
1.2 PD-L1
Programmed death ligand 1 (PD-L1), encoded by the PDCDL1 gene, is located at position p24.1.2 on human chromosome 9. PD-L1 is considered the third member of the B7 protein family, sharing 15%-20% homology with B7.1 and B7.2 proteins. PD-L1 is not only the first protein biomarker proposed for predicting immune therapy response but also the most widely used predictor so far. Tumor patients with positive PD-L1 expression generally have better overall therapeutic outcomes from immune therapy compared to those without PD-L1 expression. However, due to the heterogeneity and instability of PD-L1 expression within tumors, some PD-L1 negative patients can still benefit from PD-1/PD-L1 antibody treatments. Therefore, the application of PD-L1 as a biomarker for predicting the efficacy of immune checkpoint inhibitors (ICIs) is relatively limited.
1.3 Tumor-Infiltrating Lymphocytes (TILs)
Tumor-infiltrating lymphocytes (TILs) are one of the active anti-tumor effector cells in recent tumor immunology research, usually dominated by CD8+ T cells. Studies have shown that the lack of T cell infiltration in the tumor microenvironment is associated with non-responsiveness to PD-1 treatment in some patients. Various cancers, including non-small cell lung cancer, have demonstrated that patients with high levels of TILs have longer survival periods.
1.4 HLA
Human leukocyte antigen (HLA), also known as human MHC, is a group of closely linked genes that control intercellular recognition and regulate immune responses. HLA is located in a region of about 3.6 Mb on the short arm of human chromosome 6 at position 6p21.3 and has a high degree of genetic polymorphism. The HLA complex is the most polymorphic genetic system known in humans. HLA is widely used in research on immune-related diseases, organ and bone marrow transplantation, vaccine and drug target population screening, and tumor immunology research. Loss of HLA diversity can lead to decreased response rates to immunotherapy. HLA-B44 is a supertype of HLA capable of cross-presenting new antigens presented by other HLA subtypes, increasing HLA diversity. Studies have shown that patients with HLA-B44 positivity and high mutation levels have higher survival rates.
2. Tumor Genomic Characteristics
2.1 TMB
Tumor mutation burden (TMB) generally refers to the number of somatic non-synonymous mutations per megabase (Mb) in a specific genomic region. TMB can indirectly reflect the ability and extent of a tumor to produce new antigens and has been shown to predict the efficacy of immunotherapy in various tumors. Theoretically, the higher the number of mutations, the easier it is to stimulate the body's immune response. TMB testing can be performed in various tumors to identify patients who are more likely to benefit from immunotherapy. However, there are also many shortcomings, such as the lack of uniformity in TMB testing platforms and the fact that TMB is not entirely correlated with the response rate to immune checkpoint inhibitors. Therefore, the application of TMB still requires further research and exploration.
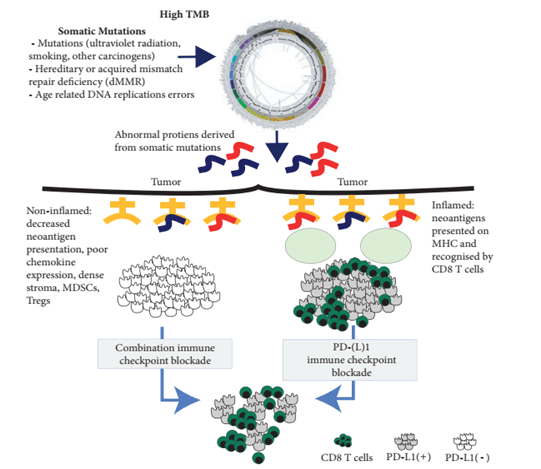
Schematic diagram of the role of TMB in tumor immunotherapy[5]
2.2 MSI
MSI (Microsatellite Instability) refers to the phenomenon of changes in the length of short, repetitive DNA sequences caused by insertion or deletion mutations during DNA replication. It is often caused by defects in the mismatch repair (MMR) system. MMR refers to the gene mismatch repair function, which is an important DNA repair mechanism that identifies and corrects errors such as base insertion, deletion, and mismatches that may occur during DNA replication or recombination, as well as repairing certain forms of DNA damage. Human mismatch repair genes are transcribed and translated to express corresponding mismatch repair proteins. Loss of MMR protein expression can result in defective mismatch repair functions in cells, leading to the accumulation of unrepaired base mismatches during DNA replication and ultimately causing MSI. MSI is categorized into high instability (MSI-H), low instability (MSI-L), and stable (MS-S); MMR is categorized into deficient mismatch repair (dMMR) and proficient mismatch repair (pMMR). Solid tumors with MSI-H/dMMR typically exhibit immunogenicity and extensive T-cell infiltration, thereby showing a good clinical response to immune checkpoint inhibitors (ICIs) therapy.
2.3 Specific Gene Mutations
Some tumors with specific driver gene mutations may not respond well to immunotherapy. For instance, patients with EGFR mutations may experience hyperprogression when treated with immunotherapy. The effectiveness of immunotherapy can be positively or negatively influenced by certain gene mutations: for example, KRAS mutations are currently considered to be positive regulators of immunotherapy, while TP53 mutations indicate a generally better outcome with immunotherapy. Furthermore, the mechanisms of mutations should be explored further, as mutations in the POLE gene may lead to different therapeutic outcomes depending on the site of mutation. PTEN mutations promote tumor progression and metastasis and are possibly associated with poorer prognosis with ICIs treatment. Research indicates that the PTEN isoform PTENα leads to T-cell dysfunction by counteracting CD8+ T cell-mediated cytotoxicity, promoting immune escape of tumor cells.
Summary
Immunotherapy, as a new approach to cancer treatment, has transformed the treatment landscape for many tumors. In recent years, with ongoing clinical research, new indications for immunotherapy have been continuously approved. Therefore, exploring more precise immunotherapy biomarkers and more effective treatment strategies are key areas for future research.
Biomarkers significantly related to immunotherapy include PD-L1 expression levels, TMB, MSI/MMR, and TILs, among which the three most established markers for immunotherapy are TMB, MSI/MMR, and PD-L1 expression. The 2019 ESMO guidelines conducted a meta-analysis (n=4186) on PD-L1/MSI/TMB and found that across all tumor types, only 2.9% of patients simultaneously exhibited TMB-H, MSI-H, and PD-L1 positivity, indicating that the consistency among PD-L1, MSI, and TMB is not high.
Overall, PD-L1, MSI-H/dMMR, and TMB-H as three independent markers can effectively predict the efficacy of immunotherapy. Currently, there are approved drugs for these three biomarkers in solid tumors. Tumor patients who are PD-L1 negative are not absolutely excluded from benefiting from immunotherapy; combined screening might allow for more precise selection of the benefiting population.
References:
[1] Pabst L, Lopes S, Bertrand B, Creusot Q, Kotovskaya M, Pencreach E, Beau-Faller M, Mascaux C. Prognostic and Predictive Biomarkers in the Era of Immunotherapy for Lung Cancer. Int J Mol Sci. 2023 Apr 20;24(8):7577.
[2] de Visser KE, Joyce JA. The evolving tumor microenvironment: From cancer initiation to metastatic outgrowth. Cancer Cell. 2023 Mar 13;41(3):374-403.
[3] Cheng Qian, Tao Ji. Research progress on efficacy markers of PD-1/PD-L1 inhibitor-based tumor immunotherapy [J]. Modern Oncology, 2023, 31(19):3688-3692.Havel J J, Chowell D, Chan T A. The evolving landscape of biomarkers for checkpoint inhibitor immunotherapy[J]. Nat Rev Cancer, 2019,19(3):133-150.
[4] Chan TA, Yarchoan M, Jaffee E, Swanton C, Quezada SA, Stenzinger A, Peters S. Development of tumor mutation burden as an immunotherapy biomarker: utility for the oncology clinic. Ann Oncol. 2019 Jan 1;30(1):44-56.
[5] Luchini C, Bibeau F, Ligtenberg MJL, Singh N, Nottegar A, Bosse T, Miller R, Riaz N, Douillard JY, Andre F, Scarpa A. ESMO recommendations on microsatellite instability testing for immunotherapy in cancer, and its relationship with PD-1/PD-L1 expression and tumour mutational burden: a systematic review-based approach. Ann Oncol. 2019 Aug 1;30(8):1232-1243.