Current location: Home > NEWS > Industry news
NEWS
PRODUCTS
Intratumoral microbiome: Unlocking the mysteries of tumor development
News source: Release time:[2024-05-30]
Microorganisms are not strange to everyone, we are dealing with microorganisms all the time, and there are countless microbial communities in our bodies. A dynamic balance of microbial flora is beneficial to human health, but an unbalanced microbial flora is harmful to the human body. Some microbiomes contribute to carcinogenesis, cancer progression, and the regulation of cancer therapy by inducing cancerous epithelial cells and chronic inflammation. At present, the role of the microbiota in tumourgenesis and clinical efficiency is mostly believed to be related to the gut microbiota. Did you know that there are microbiota in tumors? And these microorganisms may be an important driver of malignant tumor transformation. Over the past few years, research has shown that cancer tissue contains a complete community of bacteria and fungi, but how do the microorganisms in the tumor affect the development of the tumor?
More than 100 years ago, scientists detected bacteria in human tumors. Some believe this suggests that bacteria may have a local role in the tumor's microenvironment; Others point out that the bacteria are present in such low levels that it is difficult to confirm whether they really came from tumor samples or from external contamination, and their presence has not been widely noticed. Until a research paper on the microbiome, published in the journal Science in 2020, knocked on the door to explore the microorganisms in the tumor. In this study[1], the scientists did a more comprehensive analysis to understand the role of bacteria in human tumor samples. To reduce the potential problems caused by external contamination, they used a combination of different methods, not only using gene sequencing of 16S rRNA to look for bacteria, but also microscopy and cell culture to identify different bacteria living in tumors.
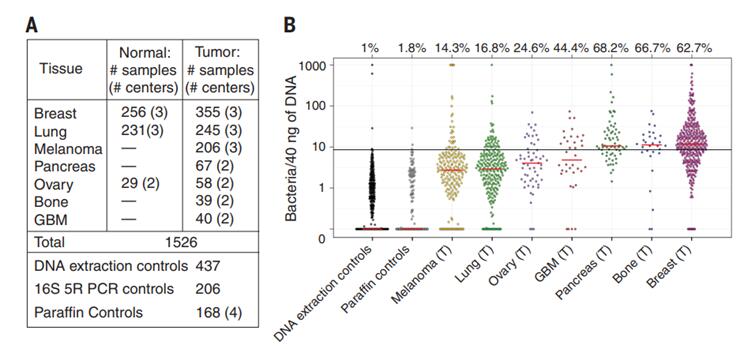
Figure 1. The researchers analyzed seven common solid tumors
The researchers analyzed seven common solid tumors, including brain, bone, breast, lung, ovarian, pancreatic, colorectal, and melanoma, with a sample size of more than 1,500 (Figure 1). The analysis confirmed that the types of bacteria were different for different tumor types. The researchers noted that different tumors have different microbiomes (Figure 2).
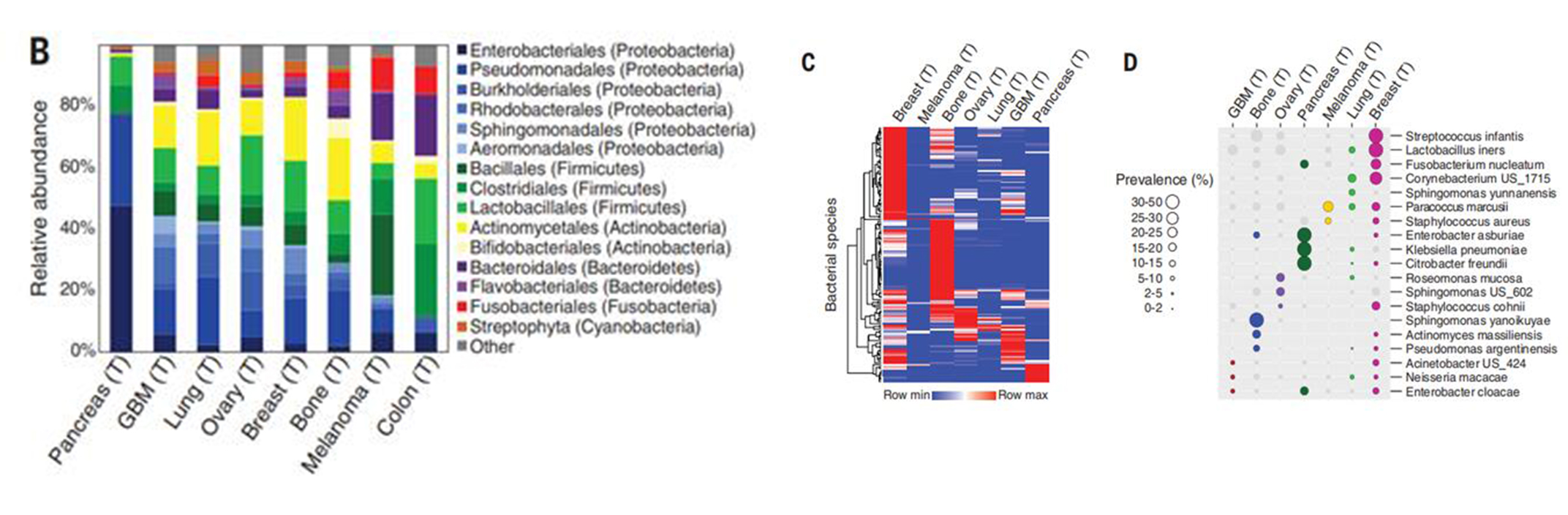
Figure 2. The types of bacteria in the tissues of different samples varied
In contrast, breast cancer tumors have a more diverse and rich microbiome (Figure 3). The bacteria were also linked to a patient's smoking history and response to immunotherapy, the researchers noted in their paper.
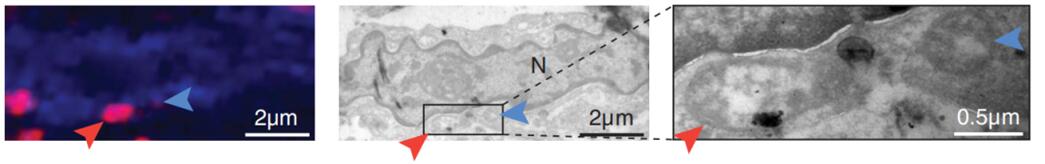
Figure 3. Bacteria observed in breast cancer samples (arrow)
In 2023, Academician Li Lanjuan's team published a review article [2] in Cell Reports Medicine, which briefly summarized the existing research methods on microorganisms in tumors, sorted out the characteristics of microorganisms in tumors (Figure 4) and their roles in various tumors, and discussed the therapeutic potential and current challenges of microorganisms in tumors.
Intratumoral microorganisms are found in esophageal (EC), pancreatic (PC), prostate (PCa), glioma, melanoma, stomach cancer (GC), renal cell carcinoma (RCC), colorectal cancer (CRC), oral squamous cell carcinoma (OSCC), liver cancer, ovarian cancer (OC), cervical cancer (CC), breast cancer (BC) and lung cancer(Table 1).
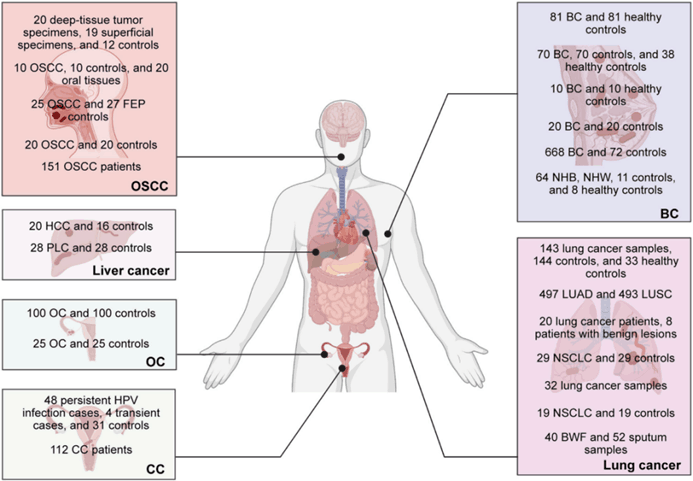
Figure 4. Characterization of intratumoral microbiota in different tumors
Table 1. Distribution of intratumoral microbiota in different tumors
Cancer | Distribution of microorganisms in tumor |
Breast cancer | The total number of bacteria in BC is reduced compared to normal tissue, and bacterial DNA load is inversely correlated with disease status. In addition, the expression level of antimicrobial response genes in tumors is lower than that in normal control tissues. Recent studies have shown that intratumoral microbiota can promote the metastasis and colonization of BC cells. |
Lung cancer | In lung squamous cell carcinoma (LUSC), acidophilus abundance is higher in smokers, and microbial differences are influenced by age and sex. In addition, acidophagous bacteria are more common in patients with squamous cell carcinoma with TP53 mutations. In lung adenocarcinoma (LUAD), Escherichia coli K-12 subchain W3110 is associated with survival and genomic alterations in older patients. There are a variety of microbial communities in the lung cancer tissues removed by surgery, almost all of which have mycoplasma presence. |
Colorectal cancer | Human CRC tissues showed enrichment of Clostridium, and the roles of nucleated clostridium mainly included immune inhibition, regulation of virulence, regulation of miRNA and lncRNA, and regulation of metabolism. The abundance of fusobacterium nucleatum in tumor tissues of CRC patients is positively correlated with the expression levels of ALPK1 and ICAM1, which can induce ALPK1 to activate the NF-kB pathway, lead to the up-regulation of ICAM1, promote the adhesion of CRC cells to endothelial cells, and promote exudation and metastasis. |
Ovarian cancer | A high percentage of HPV+ cells (42%) was observed in ovarian cancer, while only 8% of cells in normal adjacent tissue were HPV+. Viruses easily integrate into the host genome, resulting in gene inactivation and chromosomal instability that contribute to cancer progression. |
Esophagus cancer | The Cancer Microbiome Atlas (TCMA) and Cancer Genome Atlas (TCGA) analyzed the microbial characteristics of EC tissues and found that the abundance of Firmicutes increased significantly, while that of Proteobacteria decreased significantly. Some microorganisms are closely related to the subtype, stage and prognosis of EC. |
Liver cancer | A recent study using 16S rRNA MiSeq to analyze the tumor microbiome of patients with primary liver cancer (PLC) found differences in microbial populations between cancerous and matched adjacent non-tumor tissues, different histomathological subtypes, and PLC patients with different prognoses. |
Oral squamous cell carcinoma | Inflammatory bacteria are abundant in OSCC tissues, and Clostridium nucleatum and pseudomonas aeruginosa (characteristic of inflammatory flora) are associated with OSCC development. However, another study found a negative correlation between Fusobacterium nucleatum load and M2 macrophages, insensitivity to pro-inflammatory signaling, and low TLR4 signaling, resulting in a favorable prognosis. |
Prostatic cancer | In 2005, researchers isolated propionibacterium acnes from PCa tissue for the first time, and propionibacterium acnes is positively associated with prostatic inflammation. Some of the microbes in PCa samples were found to promote cancer, and surprisingly, most of the PCa tissue microbes were found to play an anti-tumor role in PCa. |
Pancreatic cancer | There are two different opinions, one thinks that the composition of pancreatic bacteria in PC patients is not significantly different from that in normal people, and the other thinks that the pancreatic tissue of PC has its own microbiota. 16S rRNA gene sequencing showed that Proteobacteria, Bacteroides and Firmicutes were the most abundant microbiota in PDAC patients. In addition to bacteria, fungal communities were found to be rich in Malassezia. The PDAC microbiome plays a carcinogenic role by influencing the host's immune system, and elimination of the PDAC microbiome could reshape the tumor microenvironment, thereby enhancing immunotherapy and potentially contributing to the treatment of PDAC. The results of another study seem to contradict these findings. Based on 16S rRNA gene sequencing, the researchers found that PDAC microbiota composition affects host immune response and disease progression. They revealed that three genera in PDAC (Saccharopolyspora, Pseudxanthomonas, and Streptomyces) can promote anti-tumor immune responses by recruiting and activating CD8+ cells. |
The difference in the distribution of microorganisms in tumor cells provides a new idea for personalized treatment. The interactions of microbial communities with tumor cells, as well as their role in disease development, provide valuable information for developing more effective treatment options. Therefore, understanding the differences in the distribution of microorganisms in tumor cells not only helps to reveal the pathogenesis of disease, but also provides a key reference for future precision medicine.
Colorectal cancer is a common malignant tumor, and its incidence ranks second among all malignant tumors and first among digestive tract malignant tumors. Early screening, early diagnosis and early treatment are of great significance to reduce the mortality and improve the prognosis of patients with colorectal cancer. A study [3] published in Nature Medicine in 2023 included fresh frozen samples from 348 primary cancer patients for a comprehensive genomic analysis, mapping a comprehensive atlas of colorectal cancer tumors, immunity and microbiome, and developing and validating a mICRoScore. This score identifies a group of patients with a good probability of survival, enabling personalized treatment.
To detect clinically relevant associations between microbiome profiles and clinical outcomes, the researchers used 16S rRNA gene sequencing data to identify microbiome features that predicted survival, comparing the relative abundance of microbiota between matched tumors and healthy colon tissue. In AC-ICAM246, the researchers set up an OS Cox regression model that selected 41 features with non-zero coefficients (associated with differential risk of death, MBR classifier). In this training cohort (ICAM246), a low MBR score (MBR<0, MBR low) was associated with a significantly lower (85%) risk of death. The researchers confirmed the association between low (risk) MBR and prolonged OS in two independent validation cohorts (ICAM42 and TCGA-COAD). There was a significant increase in clostridium in the tumors compared to the healthy samples. There was no significant difference in α diversity (the diversity and abundance of species in a single sample) between tumor samples and healthy samples, and a modest reduction in microbial diversity was observed in ICR-high tumors relative to ICR-low tumors.
 Figure 5. The correlation between MBR and OS
Figure 5. The correlation between MBR and OS
The research team identified a microbiome signature with strong prognostic value (MBR risk score). While this feature was derived from tumor samples, there was a strong correlation between healthy colorectal and tumor MBR risk scores, suggesting that the feature may capture the patient's gut microbiome composition. By combining ICR and MBR scores, it was possible to identify and validate a multigroup biomarker that predicts survival in patients with colon cancer. The study's multi-omics data set provides a resource for a better understanding of colon cancer biology that could help in the discovery of personalized treatments.
The tumor microbiome, once a neglected field, is now gradually unlocking its mystery. Their role in the occurrence and development of tumors has amazed people. However, we need to do more than just reveal the microbes in the tumor (Figure 6), but also find a way to solve this problem. A term used to describe the origin and driving force of microorganisms within a tumor. In-depth study of the origin and development of microorganisms in tumors and their relationship with tumors will help us find new ways to prevent and treat tumors. In this process, we need to have a deeper understanding of the microorganisms in the tumor, reveal its mechanism of action in the occurrence and development of tumor, and then provide new ideas for the treatment of tumor.
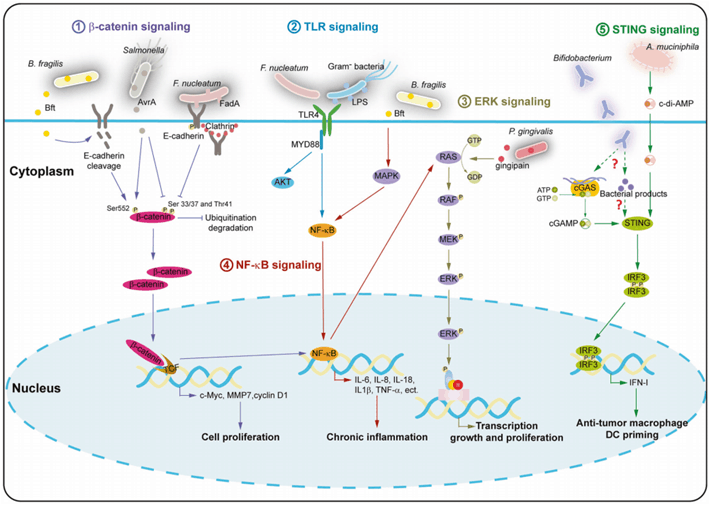
Figure 6. Signaling pathways in tumor microbiota that influence tumor biology and immune response
Looking forward to the future, the study of microorganisms in tumors will bring new hope for tumor treatment [4]. With the continuous development of science and technology, we can foresee that in the near future, microorganisms in tumors will become an important entry point for tumor treatment. By studying the microbes within tumors, we can develop targeted therapies that inhibit tumor growth and spread and improve patients' quality of life and survival. At the same time, we should also pay attention to the balance of the microbial ecosystem, maintain human health, and bring good news to cancer patients. Let's work together to uncover the mystery of the microbes in tumors and move forward for human health.
Reference
[1] Nejman D, Livyatan I, Fuks G, et al. The human tumor microbiome is composed of tumor type-specific intracellular bacteria. Science. 2020;368(6494):973-980.
[2] Xue C, Chu Q, Zheng Q, et al. Current understanding of the intratumoral microbiome in various tumors. Cell Rep Med. 2023;4(1):100884.
[3] Roelands J, Kuppen PJK, Ahmed EI, et al. An integrated tumor, immune and microbiome atlas of colon cancer. Nat Med. 2023;29(5):1273-1286.
[4] Yang L, Li A, Wang Y, Zhang Y. Intratumoral microbiota: roles in cancer initiation, development and therapeutic efficacy. Signal Transduct Target Ther. 2023;8(1):35.