Current location: Home > NEWS > Industry news
NEWS
PRODUCTS
What are the factors that affect glioblastoma targeted therapy?
News source: Release time:[2024-04-30]
1.Abstract
Glioblastoma is a highly malignant primary brain cancer with poor overall survival and limited treatment options. Recently breakthroughs in new cancer immune-related treatment strategies have stimulated interest in the recognition and elimination of malignant gliomas by the patient's tumor immune system. Here, we discuss the main factors affecting the targeted therapy of glioblastoma , and re-understand the role of targeted therapy in the treatment of glioblastoma, aiming to provide reference for the development of novel targeted therapy strategies for patients with glioblastoma.
2.Background
Glioblastoma is a neuroepithelial tumor, which accounts for 40-50% of craniocerebral tumors and is the most common intracranial malignant tumor. In the 2007 World Health Organization (WHO) classification, the major glioma groups include astroglioma, Oligodendroglioma, oligoastrocytoma, Ependymal neoplasms, and neuronal and mixed neuron-glial tumors (such as ganglioglioma)[1]. Among them, astroglioma is the most common type of glioma, accounting for about 40%. The pathological types were grade I (astrocytoma), grade II (astroblastoma), grade Ⅲ~Ⅳ(glioblastoma multiforme). Grade I to II astrocytomas are low-grade malignancies with slow onset. The manifestations of the tumors on CT and MR Are mostly solid or cystic, the boundaries are unclear, and the solid part of the tumor or cystic nodules can be strengthened. But Grade Ⅲ to Ⅳ glioblastoma multiforme has a rapid onset and is the most malignant tumor, mostly growing in the cerebral hemisphere. Due to the rapid growth of the tumor, there may be multiple necrosis and bleeding in the tumor center, and CT and MR Are significantly enhanced. With the development of glioma research in the past decades, more and more therapies for glioma have been proposed, including drug targeted therapy and immunotherapy. However, due to the influence of some biological factors, different degrees of drug resistance have been produced in the treatment of glioma. Therefore, we will explore the specific factors that affect the resistance of glioma targeted therapy, which will contribute to the new understanding of glioma immunotherapy.
3.The blood-brain barrier prevents immune cells from entering brain tissue
The blood-brain barrier refers to the barrier between the brain capillary wall and the plasma and brain cells formed by glial cells and the barrier between the plasma and cerebrospinal fluid formed by the choroid plexus. The blood-brain barrier can prevent some harmful substances and hydrophilic macromolecules from entering the brain tissue through the blood circulation, and it can also maintain the oxygen and nutrients required by the brain tissue. However, the blood-brain barrier also has a certain impact on the immune response process. For example, in the process of tumor immune response, the blood-brain barrier will prevent immune cells from entering the brain tissue, so that most immune cells cannot pass through the blood-brain barrier, and only a small number of natural immune cells can pass through smoothly, but it is not enough to cause cascaded and amplified immune response. The active immunotherapy of glioma, which can induce specific immune response to tumor by injection of exogenous antigens, has certain limitations.
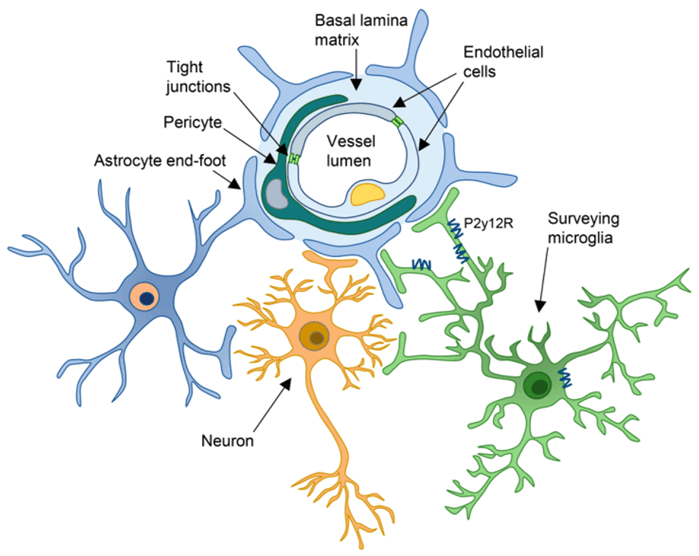
Diagram of blood-brain barrier structure[2]
4..Heterogeneity of glioma cells in different subtypes
Glioblastoma multiforme (GBM)is the most malignant and aggressive grade IV glioma. The average survival of GBM patients is about 15 months, and the 5-year survival rate is less than 5%. Based on the transcriptomic analysis of glioblastoma, gliomas are divided into proneuronal, neuronal, classical and interstitial types according to different gene mutation types, according to the Cancer Genome Atlas of the United States (TCGA) and the Chinese Population glioma Gene Atlas (CGGA). The common gene mutation types of each subtype are different, like classical type is characterized by EGFR gene mutation; The proneuronal type has the most common PDGFRA or IDH1/2 gene mutations; The interstitial type is mainly NF gene mutation, and it is similar to classical type but with higher TP53 mutation and EGFR amplification or overexpression; The markers NEFL, GABRA1, SYT1, SLC12A5 are also expressed[3,4]. Therefore, there is also a large heterogeneity of different mutation targets in different subtypes, which makes it difficult to find a treatment regimen that can cover most of the targets and antigens, which is one of the reasons for the few and poor effectiveness of current targeted and immunotherapy programs.
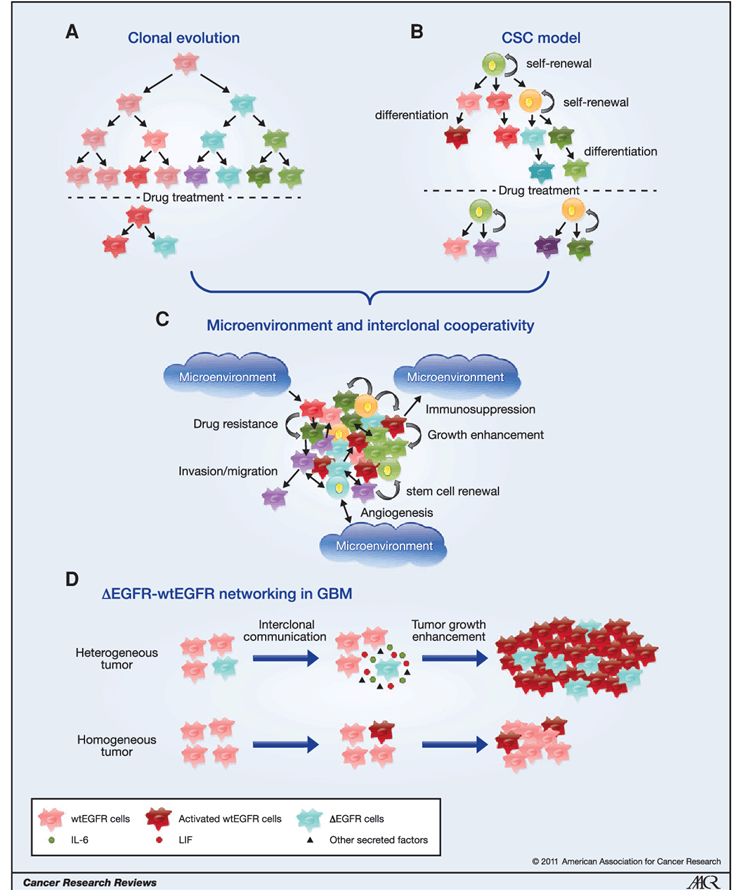
The origin and maintenance of GBM heterogeneity[5]
5.Immunosuppressive cells in the tumor microenvironment
There are CD4+ TILs and CD8+ TILs in the glioma microenvironment. According to the study, the number of CD8+ TILs in high-grade gliomas (grade III and IV) is significantly lower than that in low-grade gliomas (grade II). In contrast, the number of CD4+ TILs in high-grade gliomas is higher than that in low-grade gliomas. There is no significant difference in the number of CD4+ and CD8+ TILs between patients with grade III and grade IV gliomas. Glioma patients with higher CD4+ TILs/CD8+ TILs ratio have shorter overall survival [6]. In addition, there are a large number of regulatory T cells (TREGs) that can express the transcription factor FoxP3 in the GBM microenvironment, while there are fewer Tregs in the low-grade glioma microenvironment, indicating that the number of CD4+ TILs cells, CD8+ TILs cells and Treg cells can affect the immune response of glioma. Immune cells in the glioma microenvironment are not limited to the above types [6].
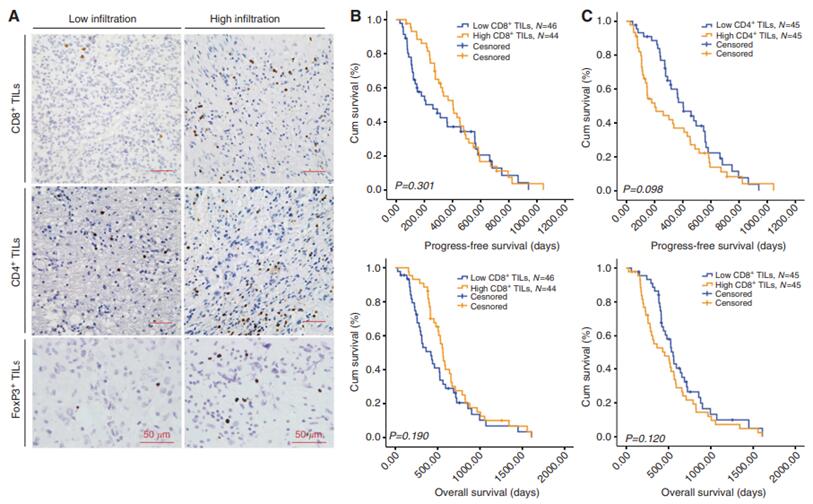
Prognostic significance of tumor-infiltrating lymphocytes (TILs) in glioblastoma[6]
6.Acquired mutations
BRAF is a rapidly accelerating fibrosarcoma (RAF) kinase activated by mouse sarcoma virus (RAS) that activates the mitogen-activated protein kinase kinase (MEK) pathway. BRAF is one of the major oncogenic driver genes in patients with pan-solid tumors. More than 90% of BRAF-activating mutations in cancer cells occur in the kinase domain of the V600 amino acid, most commonly resulting in a V600E mutation. A glioblastoma patient with BRAF V600E mutation is the first report in 2023 [7]. Patients were initially treated with BRAF+MEK combined suppression of the standard treatment for melanoma, non-small cell lung cancer, and anagenic thyroid cancer and achieved a good response, but subsequently developed therapeutic resistance through histological transformation to gliosarcoma and acquisition of carcinogenic KRAS G12D and NF1 L1083R mutations [7]. This case provides the first evidence that abnormal histological transformation of KRAS G12D/NF1 L1083R occurs simultaneously with primary BRAF V600E altered glioblastoma, a previously unrecognized mechanism of acquired resistance in the context of combined BRAF and MEK inhibition.
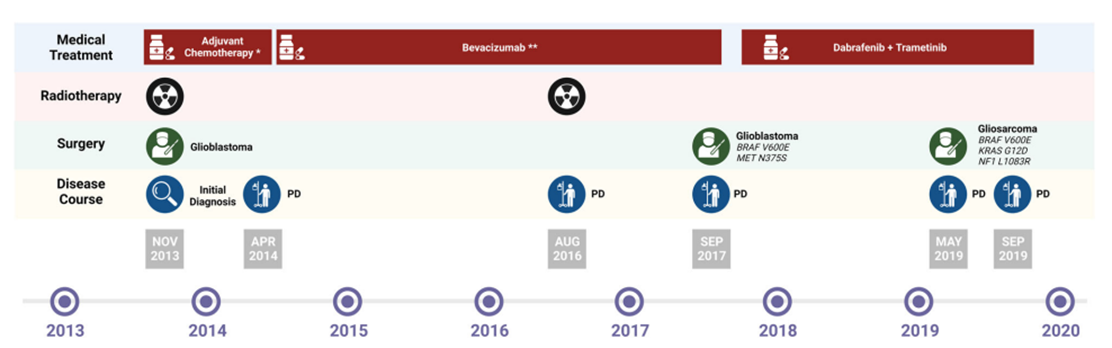
Evolution of glioma treatment[7]
7.Conclusion
At present, the main treatment methods of brain glioma are surgery, radiotherapy and chemotherapy, and there are few targeted therapy and immunotherapy options. The analysis from the perspective of drug resistance mechanism of tumor provides some reference value for the subsequent research and development of targeted and immunotherapy drugs. Finally, it is recommended that patients need to follow the doctor's advice, and regular review, so that doctors can adjust the treatment plan in time. At the same time, pay attention to rest in daily life, avoid overwork, so as not to aggravate the condition.
8.SpaceGen glioma-related gene detection
The detection of glioma gene mutation is conducive to scientific and accurate assessment of the risk of individual glioma and auxiliary typing, and the development of personalized management plans.
| Test items | Test contents | Sample types | Detection method | Reporting |
Human glioma 26 Genes Detection (NGS) | It covered 21 genes associated with glioma at the DNA level and 5 genes associated with glioma at the RNA level, and a total of 58 fusion forms are detected | Tumor tissue | NGS | 5 working days |
Glioma Genes Test (54 genes +MSI) (only services) | There are 28 glioma-related genes, 26 chemotherapy-related genes and 34 MSI loci | Tumor tissue,cerebrospinal fluid | NGS | 9 working days |
[1] Acta Neuropathol. 2007 Aug;114(2):97-109.
[2] Int J Mol Sci. 2023 May 23;24(11):9144.
[3] The Cancer Genome Atlas Program (TCGA)
[4] Chinese Glioma Genome Atlas(CGGA)
[5] Cancer Res. 2011 Jun 15;71(12):4055-60.
[6] Br J Cancer. 2014 May 13;110(10):2560-8.
[7] npj Precision Oncology (2023) 7:47