Current location: Home > NEWS > Industry news
NEWS
PRODUCTS
Application of Next Generation Sequencing in gastrointestinal stromal tumors
News source: Release time:[2024-07-25]
Background overview
Gastrointestinal stromal tumors (GISTs) are the most common mesenchymal-derived gastrointestinal tumors that originate from interstitial cells of Cajal and are more common in the stomach (60%–65%) and small intestine (20%–35%). GISTs has an insidious onset and is difficult to diagnose in the early stage, and its overall five-year survival rate is only 32%–63%. In addition, liver and peritoneal metastasis occur in 60%–85% of patients after surgery, which are the main causes of death of GISTs.
Molecular typing of GISTs
The three major molecular subtypes of GISTs are the KIT mutation, the PDGFRA mutation, and the wild type.
KIT gene: About 70% of GISTs have KIT mutation, and about 60% of GISTs in KIT are driven by point mutation or insertion-deletion mutation (insertion and/or deletion) in exon 11.
PDGFRA gene: The most common PDGFRA mutation found in all primary GISTs is the D842V point mutation within the kinase domain activation ring (encoded by exon 18), which accounts for 70% of cases of PDGFRA mutations.
Wild-type GISTs: Approximately 85% of pediatric GISTs and 10% to 15% of adult GISTs without KIT/PDGFRA gene mutations are considered wild-type GISTs.
Application of clinical Next Generation sequencing assay KIT/PDGFRA/SDH wild-type gastrointestinal stromal tumor
Clinical genome sequencing, treatment and survival results were evaluated in 20 patients with triple negative gastrointestinal stromal tumors. Genomic changes were most commonly seen in the RAS/RAF/MAPK pathway and the DNA damage response pathway. Imatinib has limited efficacy in triple-negative patients compared with KIT/PDGFRA mutant GIST.
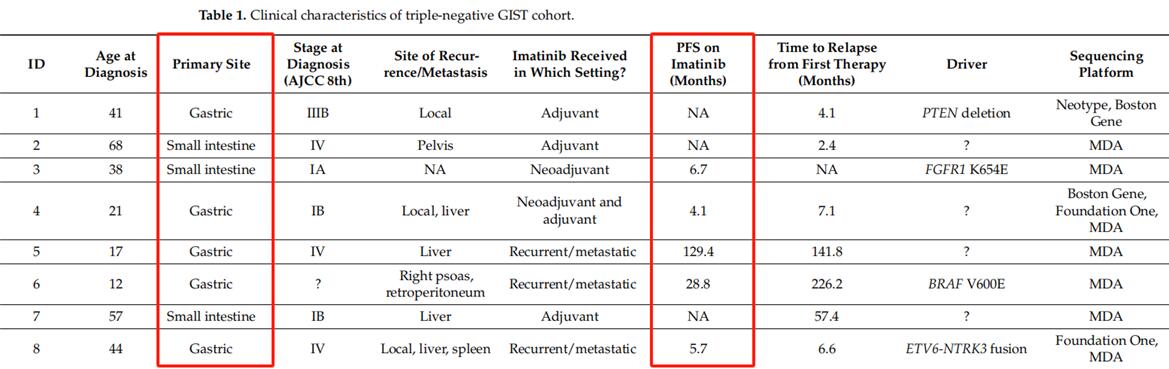
▲Clinical features of triple negative GIST cohort (not all cases presented)
All 20 patients were treated with imatinib, which is the most common first-line treatment for gastrointestinal stromal tumors, at some point in the treatment process. The mean number of rows treated was 3.0 (Median 2, range 1-9). For 13 patients with evaluable response to imatinib (ie, neoadjuvant therapy or recurrent/metastatic disease), the median PFS from imatinib treatment was 4.4 months (0.5-129.4 months).
Therapeutic response
In terms of treatment, 13 cases (65.0%) were treated with surgery as the initial treatment, and seven cases (35.0%) were treated with internal medicine. Of the 8 patients with new metastatic disease, 4 were initially treated with imatinib and 3 subsequently developed progressive disease of 2.0, 4.2 and 4.4 months.
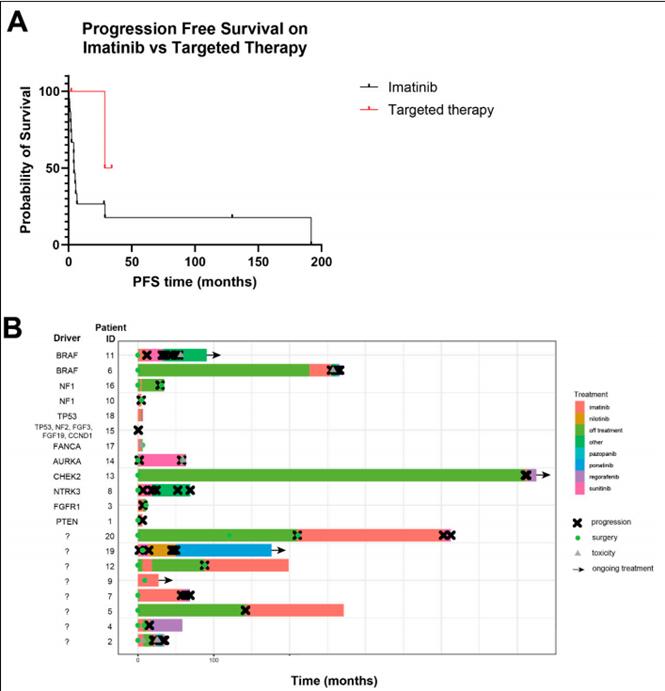
▲Bring about three negative GIST patients' reaction to treatment
Conclusion
Compared to GIST with KIT/PDGFRA mutations, this cohort had better survival outcomes but less response to targeted TKIs. Molecular analysis does reveal actionable changes in a subset of triple-negative GIST cohorts, and greater clinical benefits can be achieved with molecular matching therapy compared to typical TKIs. In patients without identifiable changes on the next-generation sequencing panel, more comprehensive genetic and epigenetic assessments may be desirable to establish unknown tumorigenesis.
References
[1] 2024 CSCO guidelines for gastrointestinal stromal tumors
[2] Cancers 2024, 16, 1707. https://doi.org/10.3390/cancers16091707
Statement: This article is only for sharing, if it involves copyright issues, please contact us as soon as possible, we will correct the first time, thank you!