Current location: Home > NEWS > Industry news
NEWS
PRODUCTS
Do patients with hepatobiliary tumors really not need genetic testing?
News source: Release time:[2024-05-17]
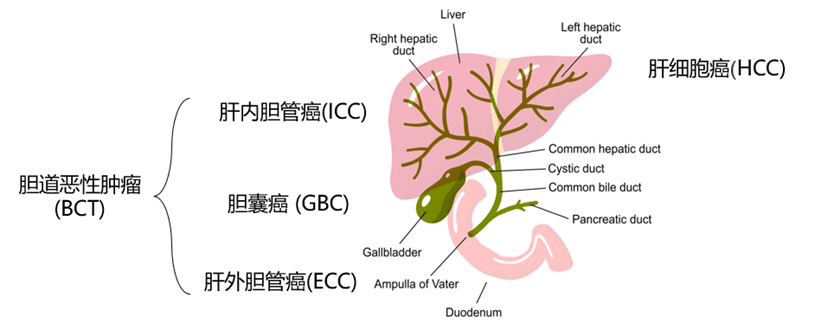
Hepatobiliary tumors include primary liver cancers (PLC) such as hepatocellular carcinoma (HCC), intrahepatic cholangiocarcinoma (ICC), and mixed carcinoma (HCC-ICC), as well as bile duct cancer (CCA) and gallbladder cancer (GBC), which are malignant tumors originating in the bile ducts.
Treating liver cancer is challenging, with a poor prognosis. The incidence-to-mortality ratio is as high as 1:(0.8~0.9). In North American countries and regions, the 5-year survival rate is 15%~19%, whereas in our country, it is only 12.1% [1]. Although malignant biliary tumors are rare, the vast majority of these tumors are adenocarcinomas, which are highly invasive and often diagnosed at a late stage with an extremely poor prognosis. The 5-year survival rate is less than 5%[1].
Currently, although many molecular targeted therapies and immunotherapies have been approved for liver cancer, some first-line targeted drugs (sorafenib, lenvatinib) and second-line targeted drugs (regorafenib, cabozantinib) are anti-angiogenic agents. These drugs are multi-targeted without clear efficacy targets, and gene testing is not recommended for them. For other biliary tumors, chemotherapy remains the primary first-line treatment, so some clinicians do not recommend gene testing for patients with liver and biliary tumors.
So, do patients with liver and biliary tumors not need to undergo gene testing?
NO
In the clinical diagnosis of liver and biliary cancers, histopathological and cytological diagnoses are the gold standards for confirming these cancers. Due to the complexity and difficulty of interpreting histopathological results, there may be a certain false-negative rate. Guidelines and consensus suggest that when necessary, genomic and other molecular marker testing should be performed to aid in differential diagnosis [2-3].
In recent years, there have been some advancements in targeted and immunotherapy for liver and biliary tumors, with more and more targeted and immunotherapy drugs gradually being used for the treatment of these cancers.
01
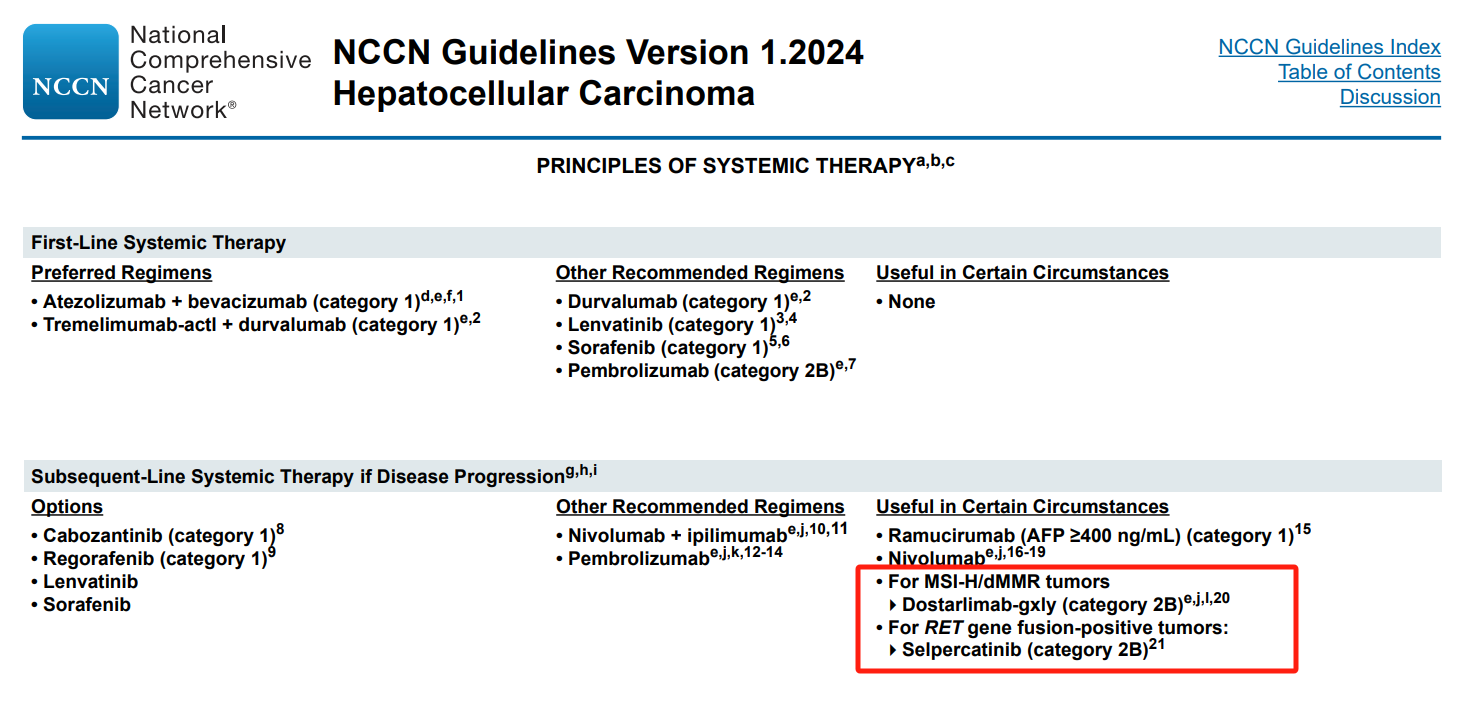
Before targeted therapy for hepatocellular carcinoma (sorafenib, lenvatinib, regorafenib, etc.), routine genetic screening to predict efficacy is not recommended. However, based on the clinical situation or clinical trials, patients may undergo genetic testing for RAS, MET, HRD, VEGFA, etc., to provide a reference for treatment post-resistance and combination therapies in hepatocellular carcinoma.
02
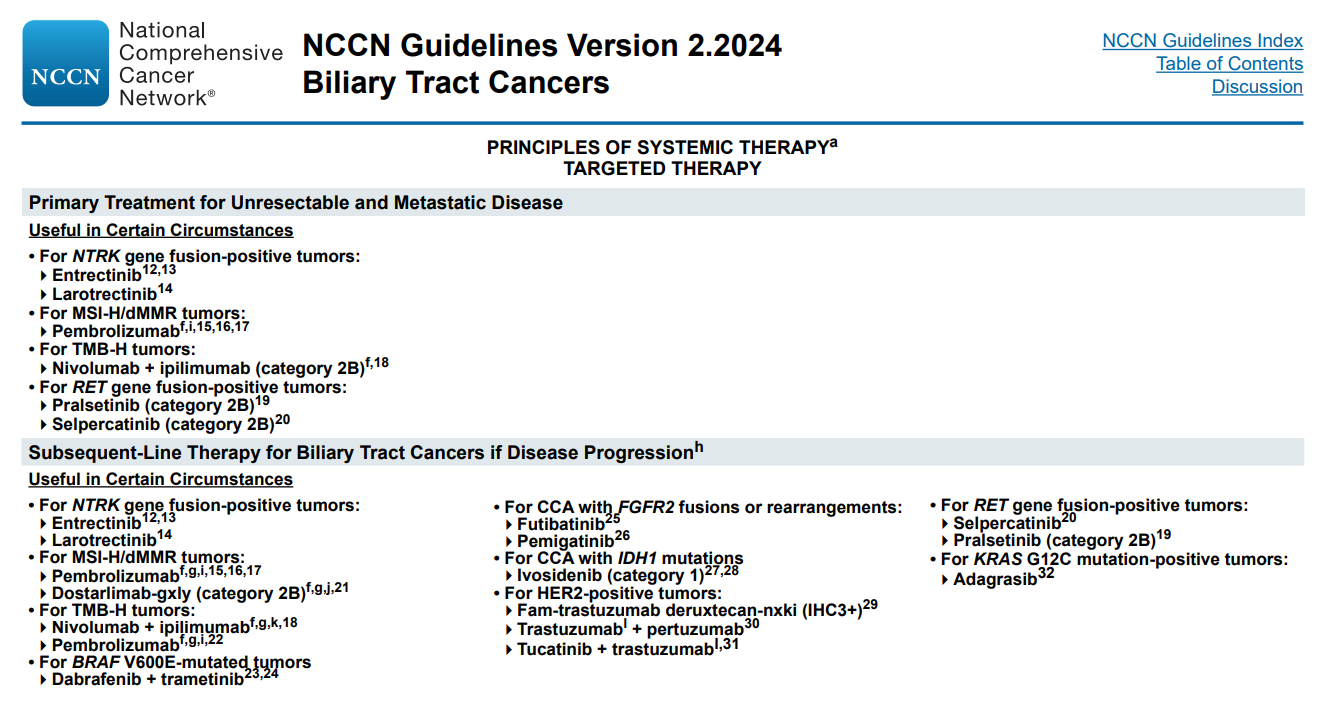
In clinical practice, based on the actual situation or clinical trials, genetic testing for FGFR, ERBB2, BRAF, IDH, HRD, PI3K/mTOR, FGF19, and other genes can be attempted in CCA/gallbladder cancer patients to explore new personalized targeted therapy schemes for patients.
03
Before immunotherapy for liver and bile duct tumors (PD-1 antibodies, PD-L1 antibodies, CTLA-4 antibodies), it is not recommended to use routine genetic screening to select the preferred population for immunotherapy. However, based on the clinical situation or clinical trials, patients can undergo histological or serological testing for PD-L1, TMB, MSI, etc., to explore effective molecular diagnostic markers for immunotherapy.
Due to the limited targets and low mutation frequency in liver and bile duct tumors, as well as the particularity of related drugs, not all patients need genetic testing. However, if one wants to try treatment options outside the guidelines, genetic testing may offer a "lifeline."
SpaceGen is committed to providing comprehensive solutions for personalized precision diagnosis of tumors, including early screening, disease diagnosis, personalized medication guidance, and efficacy monitoring. Based on the different testing needs of liver and bile duct tumor patients, we recommend the following testing packages:
| Product | Targeted therapy related genes | Immunotherapy TMB | Immunotherapy MSI | Sample type | Genetic susceptibility genes |
Human FGFR1/2/3 Gene Fusions Detection Kit | √ | ||||
Hereditary Cancers Panel | √ | √ | √ | √ | √ |
Different tests for patients with hepatobiliary tumors>>>>>
References
[1] The Lancet Global Health,2018, 6(5):e555-e567.
[2] CSCO Guidelines for Diagnosis and Treatment of Primary Liver Cancer (2023)
[3] CSCO Guidelines for Diagnosis and Treatment of Biliary Tract Malignancies (2023)