Current location: Home > PRODUCTS > Next Generation Sequencing Series Products
PRODUCTS
Lynchcan®
CE-IVD
GENE MUTATION AND TUMOR
Lynch syndrome (LS) is a high penetrance, autosomal dominant susceptibility syndrome.The pathogenesis of LS is single allele germline mutation of mismatch repair genes (especially MLH1, MSH2, MSH6 or PMS2), or epigenetic silencing of MSH2 caused by germline deletion in adjacent EPCAM gene. LS can cause colorectal cancer and tumors in other organs (including endometrium, ovary, stomach, small intestine, liver and gallbladder, upper urethra, brain and skin). The cancer risk of LS carrier is higher than that of normal people.
NCCN guidelines recommend that all patients with newly diagnosed colorectal cancer should be screened by immunohistochemistry or microsatellite instability detection of four MMR proteins (MSH2, MSH6, PMS2, MLH1) in tumor tissue.For dMMR patients identified by immunohistochemistry, it is suggested to further detect germline mutations of protein expression deletion genes.For patients with microsatellite instability determined by MSI method, germline mutation detection of MSH2, MSH6, PMS2, MLH1 and EPCAM genes is recommended.
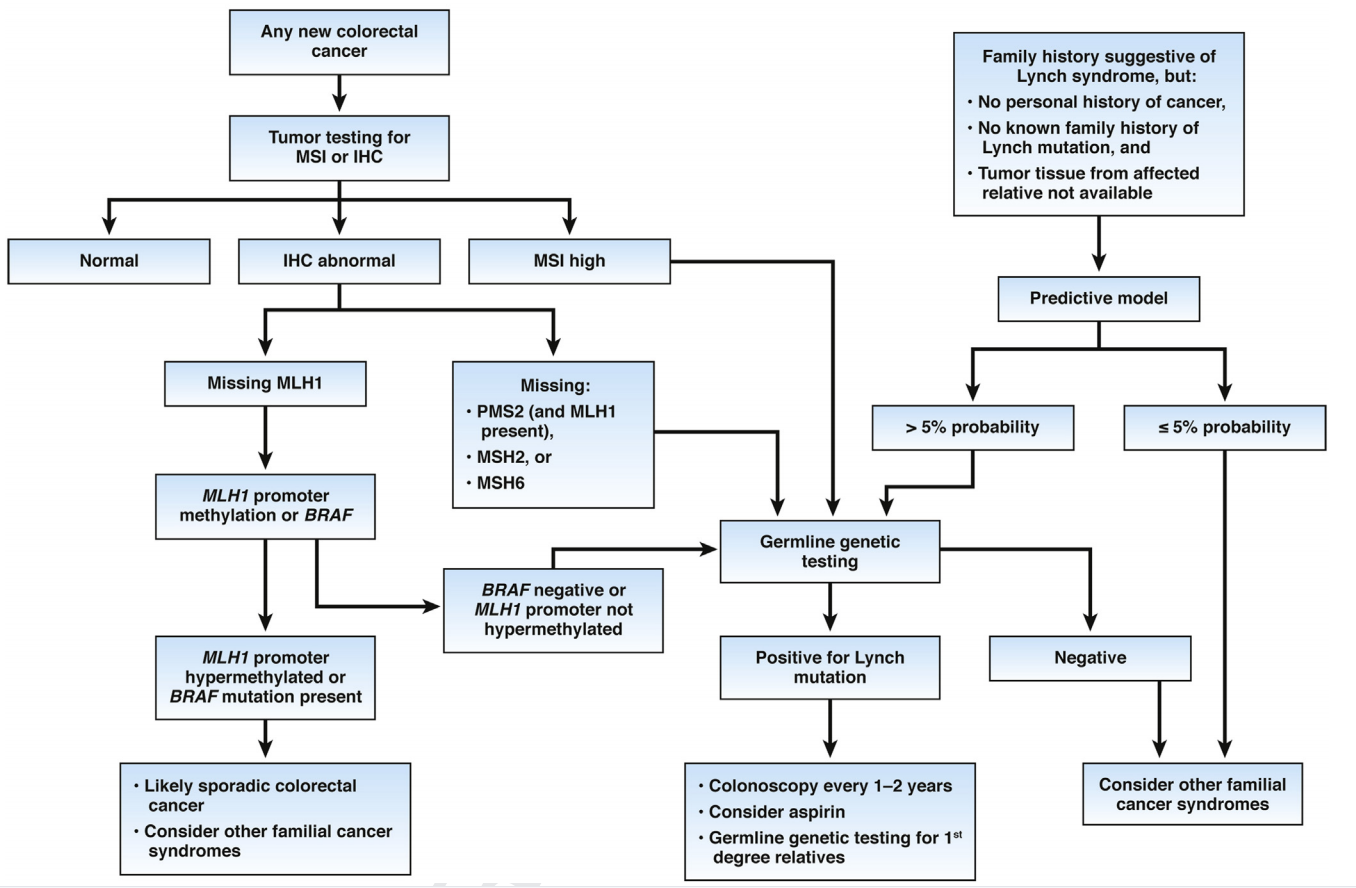
American Gastroenterological Association Institute Guideline on the Diagnosis and Management of Lynch Syndrome
DETECTED GENES
| Gene | Covering Exons |
| MSH2, MSH6, PMS2, MLH1, EPCAM | Coding region, exon intron junction region |
| BRAF | V600E |
PRODUCT INFORMATION
| Product Name | Core Technology | Pack Size | Instruments Validated | Sample Type |
Lynchcan® Human Lynch Syndrome Gene Detection Kit | RingCap® | 16 Tests/kit 32 Tests/kit | Illumina MGISEQ | Tumor tissue Peripheral blood |
DETECTION SIGNIFICANCE
1. Confirmed diagnosis of LS is based on germline testing.
2. Mutant BRAF V600E suggests sporadic CRC.
APPLICABLE PEOPLE
dMMR/MSI-H tumor patient.
The relatives of confirmed LS patient.
FEATURES & ADVANTAGES
1. Ease of Use: Based on the patent technology RingCap®, Library preparation in 2 steps.
2. Fast Results: The library preparation takes only 3.5 hours.
3. High Sensitivity: Sequencing depth can be above 500 x, the sensitivity can reach up to 5%.
4. Comprehensive Coverage: A single test for mutations in the coding region, exon-intron junction region of five genes associated with Lynch syndrome, with broad coverage.
Detection of BRAF V600E as a basis for exclusionary diagnosis of Lynch syndrome.
DETECTION PROCESS
Nucleic Acid Extraction
Library Preparation (3.5 hours total time)
Sequencing
Auto-data Analysis
Report