Current location: Home > PRODUCTS > Next Generation Sequencing Series Products
PRODUCTS
(Next Generation Sequencing)
CE-IVD
GENE MUTATION AND TUMOR
Pan cancer driver gene mutation detection panel aims at the companion diagnostic genes approved by FDA and recommended by NCCN guidelines, covering 56 genes related to cancer treatment and prognosis, including 3000 cosmic mutation sites, so as to realize low-cost, high sensitivity and high-throughput gene detection of tumor tissue and circulating free DNA of cancer patients.
Cancer is a complex polygenic disease caused by the gradual accumulation of gene mutations. When the genes regulating cell growth are mutated or damaged, the cells lose control and proliferate and differentiate disorderly and infinitely, leading to the occurrence of malignant tumors.
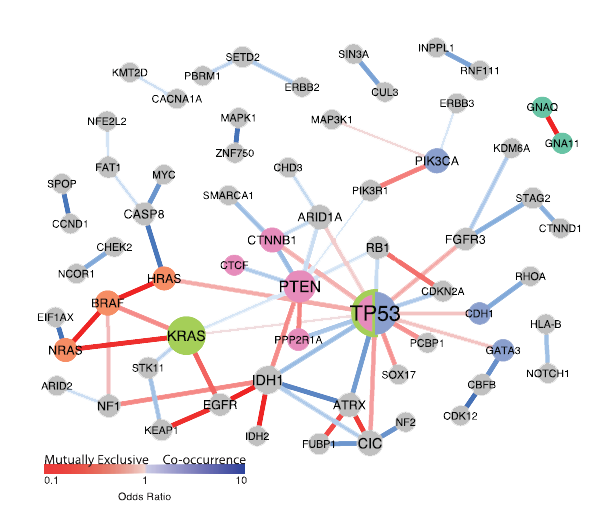
Cell. 2018 Apr 5;173(2):305-320.e10.
Tumor precise diagnosis and treatment products provide important reference basis for precise drug treatment, molecular typing and efficacy evaluation by accurately analyzing the unique gene mutation information of each tumor patient.
About NCI-MATCH
NCI-MATCH, also known as MATCH, is a precision medicine cancer treatment clinical trial. In this trial, people with cancer are assigned to receive treatment based on the genetic changes found in their tumors through genomic sequencing and other tests. Genomic sequencing is a laboratory method that is used to determine the genetic makeup of cancer cells. People whose tumors have genetic changes that match one of the treatments in the trial may receive that treatment if they meet other eligibility criteria. The trial seeks to determine whether treating cancer based on these specific genetic changes is effective, no matter the cancer type.
PRODUCT INFORMATION
| Product Name | Core Technology | Pack Size | InstrumentsValidated | Sample Type | |
Human Pan-Cancer Drive Gene Mutations Detection Kit | RingCap® | 16 Tests/Kit 32 Tests/Kit | Ion Torrent Illumina MGISEQ | Tumor tissue samples Peripheral blood Pleural effusion | |
DETECTION CONTENT
Gene Mutation | EGFR | KRAS | BRAF | PIK3CA | HER2 |
| MET | RB1 | ALK | ERBB4 | HNF1A | |
| MPL | SMAD4 | ATM | FBXW7 | IDH1 | |
| SMO | CDH1 | FGFR2 | JAK3 | NRAS | |
| STK11 | CSF1R | FLT3 | KDR | TP53 | |
| ABL1 | GNAS | PTPN11 | AKT1 | GNAQ | |
| MLH1 | RET | APC | EZH2 | HRAS | |
| NOTCH1 | SMARCB1 | FGFR1 | JAK2 | NPM1 | |
| SRC | CDKN2A | FGFR3 | IDH2 | PDGFRA | |
| CTNNB1 | GNA11 | KIT | PTEN | VHL | |
| Gene Fusion | ALK | ROS1 | RET | NTRK1 | NTRK2 |
| NTRK3 | |||||
Gene Amplification | HER2 | MET | |||
| Note: HER2 and MET include copy number variation detection. | |||||
DETECTION SIGNIFICANCE
Personalized Medication: Before using targeted drugs for malignant tumor patients, gene testing can be carried out to assist clinicians in judging the sensitivity of patients to drugs and evaluating the prognosis of patients.
FEATURES & ADVANTAGES
1.Ease of Use: With patented RingCap® technology, Library preparation in 2 steps.
2.Fast Results: The library preparation takes only 3.5 hours.
3.High Sensitivity: The sensitivity can reach up to 1%.
3.Comprehensive Coverage: 3000 kinds of cosmic mutation sites can be detected at one time.
DETECTION PROCESS
1.Nucleic Acid Extraction
2.Library Preparation (3.5 hours total time)
3.Sequencing
4.Auto-data Analysis
5.Report