Current location: Home > PRODUCTS > Next Generation Sequencing Series Products
PRODUCTS
Typesen®
(CE-IVD)
Endometrial Carcinoma is the most common malignant tumor of the female reproductive system in developed countries.Factors related to endometrial cancer: 1. Obesity> 23kg of normal weight, the risk increases by 10 times; 2. Women who have not given birth and infertility;3. Patients with functional ovarian tumors (granulocytoma); 4. Long-term use of females hormones; 5. Genetic factors, family history of colon cancer (lynch syndrome).
In 2013,The Cancer Genome Atlas (TCGA) studied 373 cases of Endometrial Carcinoma, Using genomics , transcriptomics and proteomics methods, by analyzing mutation spectrum, microsatellite instability (MSI), somatic cell copy Number changes (SCNAs), etc. TCGA divided Endometrial Carcinoma into four categories:(1)POLE ultramutated. (2) microsatellite instability-High(MSI-H) (3) copy number low(CN-L). (4)copy number high (CN-H).
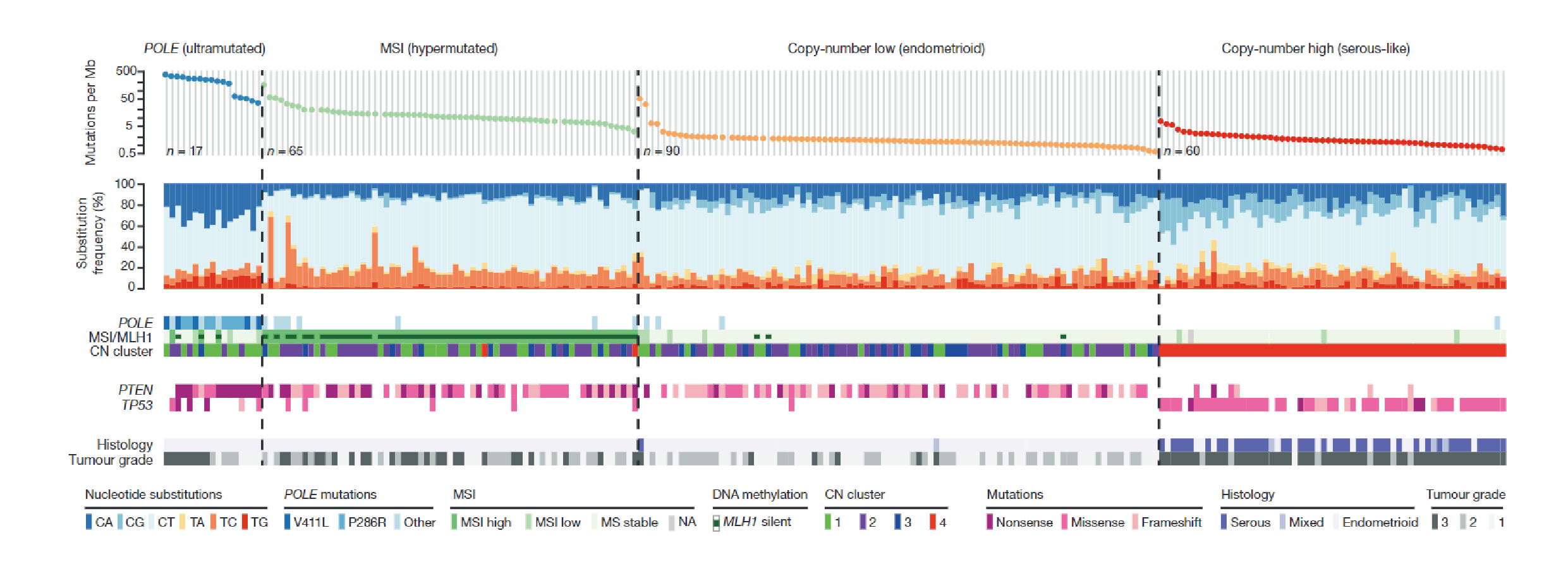
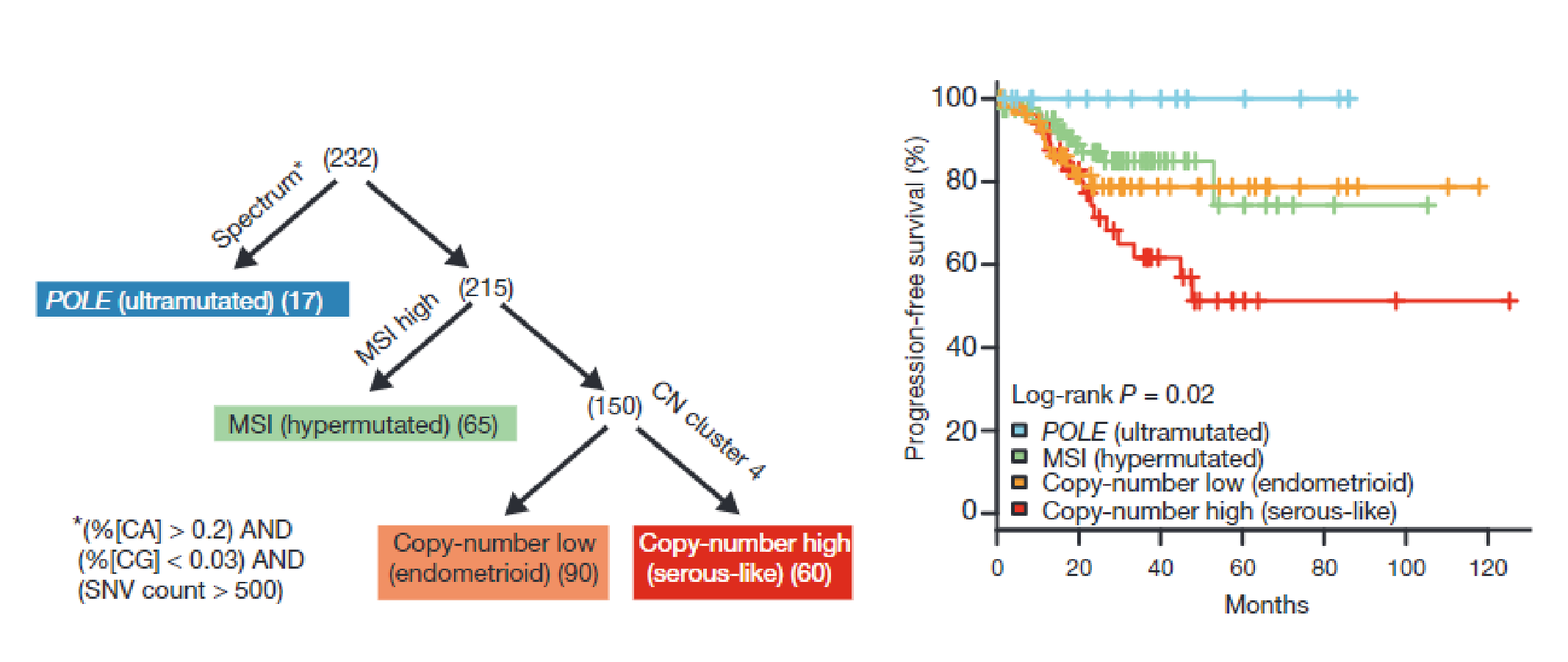
Nature. 2013 May 2; 497(7447):67-73.
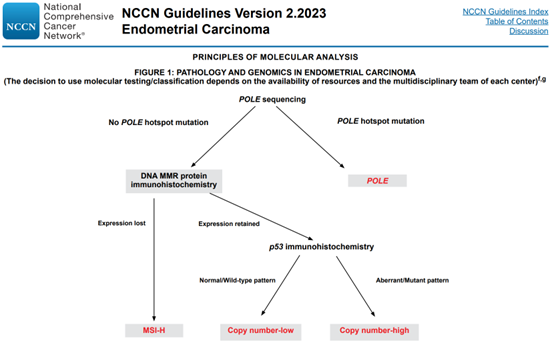
NCCN RECOMMENDS MOLECULAR CLASSIFICATION DETECTION
DETECTION ITEM
The test kit detect 12 genes (POLE, TP53, MLH1, MSH2 , PMS2, MSH6, EPCAM, PTEN, KRAS, CTNNB1, PIK3CA, HER2) and MSI, related to the molecular classification of Endometrial Carcinoma.
DETECTION SIGNIFICANCE
POLE Ultramutated | If the surgical stage is I/II,observation is preferred. |
MSI-H | Intermediate prognosis, sensitive to treatment with immune checkpoint inhibitors, but current evidence is limited to advanced and recurrent cases. It is helpful for the screening of Lynch syndrome. |
Copy Number Low (CN-L) | Intermediate prognosis, better outcomes in patients who recieved fertility-sparing therapy and are simultaneously with progesterone receptor–positive. |
Copy Number High (CN-H) | Unfavorable prognosis, requires intensified adjuvant treatment, highly significant benefit from CTRT. |
PRODUCT INFORMATION
Product Name | Core Technology | Pack Size | Instruments Validated | Sample Type |
Typesen® Human Endometrial Cancer Molecular Classification Detection Kit | RingCap® | 16 tests/kit 32 tests/kit | Illumina MGISEQ | Tumor tissue |
FEATURES & ADVANTAGES
1. Scientific and Rigorous: Molecular classification for Endometrial Carcinoma is included in NCCN and CSCO guidelines.
2. Fast Result: Based on the independent patent technology RingCap,library preparation takes only 3.5h in 2 steps.
3. High Sensitivity: The sequencing depth is up to 5000X and the sensitivity can reach as low as 1%.
4. Comprehensive Coverage: Detects 12 genes and MSI related to the molecular classification of Endometrial Carcinoma at one time.
DETECTIONPROCESS
1. Nucleic Acid Extraction
2. Library Preparation (3.5 hours total time)
3. Sequencing
4. Auto-data Analysis
5. Reporting