Current location: Home > PRODUCTS > Next Generation Sequencing Series Products
PRODUCTS
(CE-IVD)
The susceptible genes for breast cancer, including BRCA1 and BRCA2, are important tumor suppressor genes that play a role in homologous recombination repair (HRR) of DNA damage. Mutations in the BRCA1/2 genes can lead to homologous recombination deficiency (HRD), resulting in a significant increase in genomic instability. The variant status of the BRCA1/2 genes is of significant importance in genetic risk assessment, treatment selection, and prognosis determination for related tumors such as ovarian cancer, breast cancer, pancreatic cancer, prostate cancer, etc. The BRCA1/2 genes have relatively long sequences and diverse forms of mutations, with mutation sites scattered throughout the entire length of both genes. Therefore, BRCA1/2 gene testing must simultaneously cover the coding regions and adjacent boundary regions (ideally within ±20 bp) [1].
BRCA AND HEREDITY
Germline mutations in the BRCA1/2 genes originate in reproductive cells, significantly increasing the risk of developing breast cancer, ovarian cancer, and other related tumors [1]. About 10% of breast cancer patients [2-3], 10-15% of ovarian cancer patients [4], and 10% of prostate cancer patients [5] are caused by germline mutations in the BRCA1/2 genes.
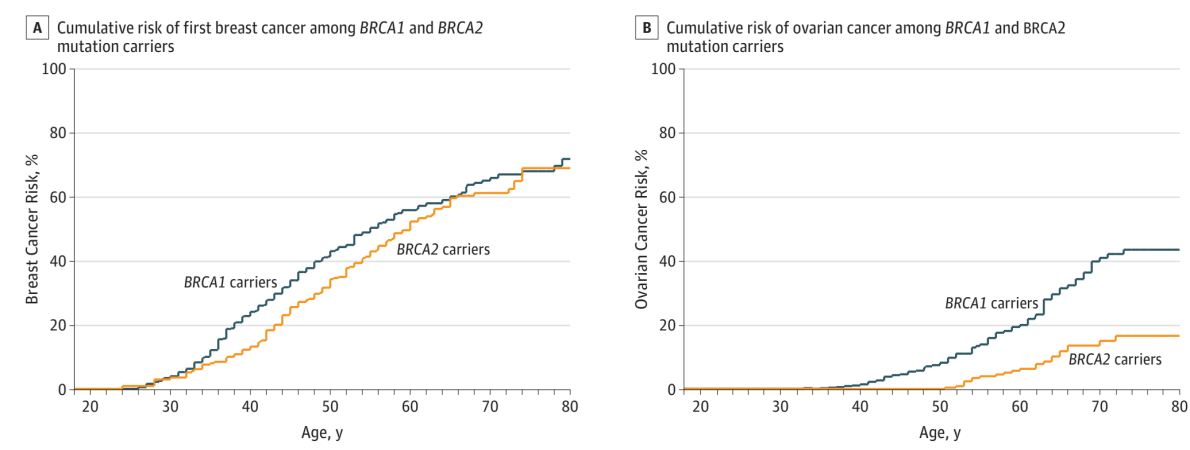
JAMA. 2017 Jun 20; 317(23): 2402-2416.
Figure A: BRCA1 and BRCA2 mutation carriers have a cumulative breast cancer risk of 72% and 69% by the age of 80, respectively;
Panel B: BRCA1 and BRCA2 mutation carriers have a cumulative ovarian cancer risk of 44% and 17% by age 80, respectively [6].
BRCA AND TREATMENT
The variant status of BRCA1/2 genes is closely related to the effectiveness of Poly(ADP-ribose) polymerase (PARP) inhibitors. In recent years, the FDA and NMPA have approved a variety of PARP inhibitors for the treatment of related tumors [1]. Approximately 20% of ovarian cancer patients carry BRCA1/2 gene mutations [7], making them the population that benefits the most from maintenance treatment with PARP inhibitors. Furthermore, multiple PARP inhibitors have been approved for various indications in breast cancer, prostate cancer, and pancreatic cancer. These indications all require BRCA1/2 gene testing as a companion diagnostic.
PRODUCT INFORMATION
Detect exon 1 of BRCA1/2 gene + full CDS region + exon and intron junction region ± 20bp.
| Product Name | Core Technology | Pack Size | Instruments Validated | Sample Types |
Brcasen® BRCA1/2 Gene Mutations Detection Kit | RingCap® | 16 tests/kit 32 tests/kit | Ion Torrent Illumina MGISEQ | Tumor tissue |
Peripheral blood Oral swab Saliva |
DETECTION SIGNIFICANCE
By detecting whether probands or high-risk groups carry BRCA1/2 gene germline mutations, it provides a basis for the diagnosis and subsequent diagnosis and treatment of hereditary tumors;
Select malignancy patients for the treatment with PARPi based on BRCA mutations.
FEATURES & ADVANTAGES
Simple Operation: Based on the independent patent technology RingCap®, library preparation need only 2 steps and takes only 3.5 hours.
High Sensitivity: Detect gene mutations as low as 5% in 10ng DNA samples.
Comprehensive Coverage: Since there are no high-frequency mutation hotspots in the BRCA1/2 genes, sequencing of both the coding regions and adjacent boundary regions ensures comprehensive coverage in the testing process.
Rigorous and professional: Comprehensive and strict quality control standards, automated analysis + double verification by a professional team, truly achieving comprehensive and accurate interpretation.
DETECTION PROCESS
1.Nucleic Acid Extraction
2.Library Preparation (3.5 hours total time)
3.Sequencing
4.Auto-data Analysis
5.Reporting
REFERENCES
[1] BRCA1/2 Data Interpretation Chinese Expert Consensus (2021 Edition)
[2] Chinese Expert Consensus on Clinical Diagnosis and Treatment of Hereditary Tumors (2021 Edition) - Hereditary Breast Cancer
[3] Int J Cancer. 2017 Jul 1; 141(1): 129-142.
[4] Chinese Expert Consensus on Clinical Diagnosis and Treatment of Hereditary Tumors (2021 Edition) - Hereditary Ovarian Cancer
[5] JCO Precis Oncol. 2017 Jul;2017:PO.17.00029.
[6] JAMA. 2017 Jun 20; 317(23): 2402-2416.
[7] Nature. 2011 Jun 29; 474(7353): 609-15.