Current location: Home > PRODUCTS > Next Generation Sequencing Series Products
PRODUCTS
Cardiotoxicity Prediction of Breast Cancer Detection Kit
(Next Generation Sequencing)
CE-IVD
Cardiotoxicity of Chemotherapy
The toxicity of breast cancer chemotherapy to the heart is mainly derived from anthracyclines, and the cardiotoxicity caused by anthracyclines is usually progressive and irreversible. After receiving anthracycline treatment, 6% of patients may develop clinical cardiotoxicity, the incidence of subclinical cardiotoxicity was 18% [1]. The first use of anthracyclines may cause damage to the heart, which is cumulative and affects anti-tumor treatment as well as the quality of life of patients. Chronic and delayed cardiotoxicity of anthracyclines is related to its cumulative dose, therefore limit the cumulative dose of anthracyclines can reduce the incidence of cardiotoxicity. Patients with high risk factors for heart damage should fully be assessed the risk of cardiotoxicity for adjusting the medication plan and dosage before selecting the drug. High-risk patients should avoid the use of anthracyclines [2].
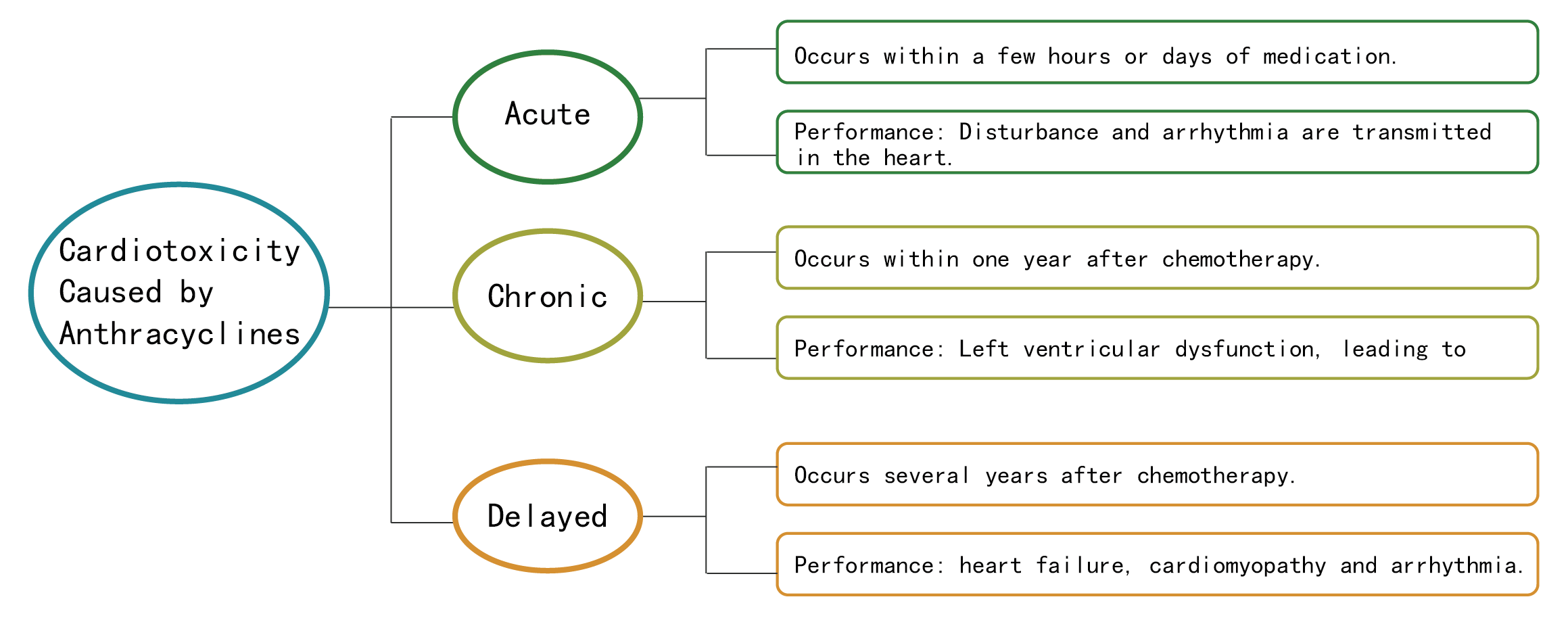
Cardiotoxicity Distribution of Anthracyclines [3]
Trastuzumab combined with chemotherapy drugs, especially anthracycline chemotherapy drugs, can increase myocardial damage, even heart failure can occur in severe cases. Therefore, trastuzumab combined with anthracyclinechemotherapy is not recommended for patients with recurrent and metastatic breast cancer[2].
[1] Am J Cardiol. 2013 Dec 15;112(12):1980-4.
[2] Chinese Society of Clinical Oncology Breast Cancer Diagnosis and Treatment Guidelines 2021
[3] Guidelines for Prevention and Treatment of Cardiotoxicity of Anthracyclines (2013 Edition)
Personalized Medication
There is no absolute "safe dose" for anthracyclines, which may be due to individual differences, that is, the differences in the genes related metabolism of anthracyclines may lead to different susceptibility to anthracyclines [3]. Genetic mutations prone to cardiotoxicity caused by anthracyclines have clearly included genes involved in drug transport and metabolism, energy utilization, oxidative stress, apoptosis and cytoskeleton regulation [4]. Genetic testing of individual patients is recommended before treatments for better selection of treatment options, maximizing the therapeutic effect and avoiding the risk of cardiotoxicity.
[4] Nat Rev Clin Oncol. 2015 Dec;12(12):718-31.
Detection Item
Detection of 38 genes related to breast cancer treatment of cardiotoxicity: UGT1A6, FANCD2, ABCC5, PIK3R1, SLC22A7, VEGF6, GSTA1, SLC22A16, ABCB1, CYP3A5, CYP3A4, SLC28A3, ESR2, AKR1C3, CYP2C8, ABCC2, CAT, GSTP1C8 , SLCO1B3, RARG, SLC22A17, TCL1A, MRP1, ABCC1, NQO1, CYBA, HER2, RAPTOR, CYP2B6, CBR1, NCF4, RAC2, CYP2D6, CBR3, NOS3, ESR1, CELF4, and DPD, covering 56 genetic variants sites.
Performance Parameter
Product Name | Core Technology | Product Specifications | Compatible Instrument | Sample Type | |
Cardiotoxicity Predictionof Breast Cancer Detection Kit | RingCap® | 16 tests/kit 32 tests/kit | Ion Torrent, Illumina | peripheral blood | |
Detection Signification
For patients with cardiovascular disease who have received anthracycline chemotherapy or radiation therapy, older than 65 years old with high risk factors for heart damage, the risk of cardiotoxicity should be fully assessed before chemotherapy drugs are used for adjusting medication regimen and dose.
ProductHighlights
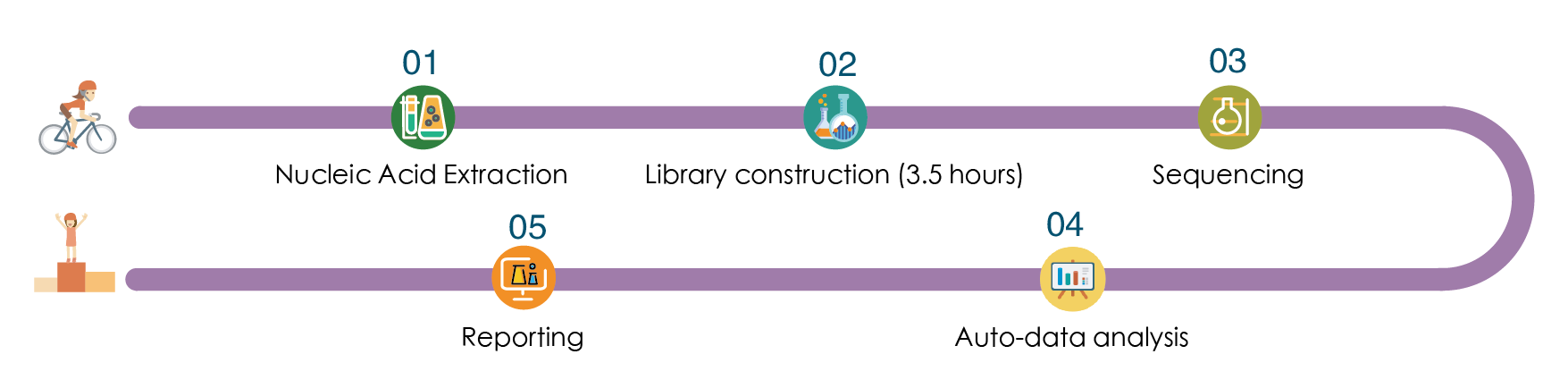
Product Highlights:Based on the independent patent technology RingCap®, library construction can be finished in only 2 steps.
Rapid Detection:The library construction takesonly 3.5 hours, only 2 working days from sampling to reporting.
Compreh ensive Detection:Covers common genes and sites related to breast cancer treatment of cardiotoxicity.
Accurate and Reliable:Use specific primers to amplify and capture the target fragment.
Detection Process