Current location: Home > PRODUCTS > Next Generation Sequencing Series Products
PRODUCTS
Genecan®
(CE-IVD)
GENE MUTATION AND TUMOR
The risk of colorectal cancer is closely related to lifestyle. Increased intake of food from animals and overweight due to sedentary are associated with the risk of colorectal cancer. Other risk factors include alcohol intake, smoking, eating red or processed meat. Proper supplementation of calcium, cereals, fiber and dairy products can reduce its risk. In recent years, with the improvement of social and economic level. The overall incidence rate and mortality rate of colorectal cancer in China showed a significant upward trend, and the proportion of colorectal cancer increased year by year.
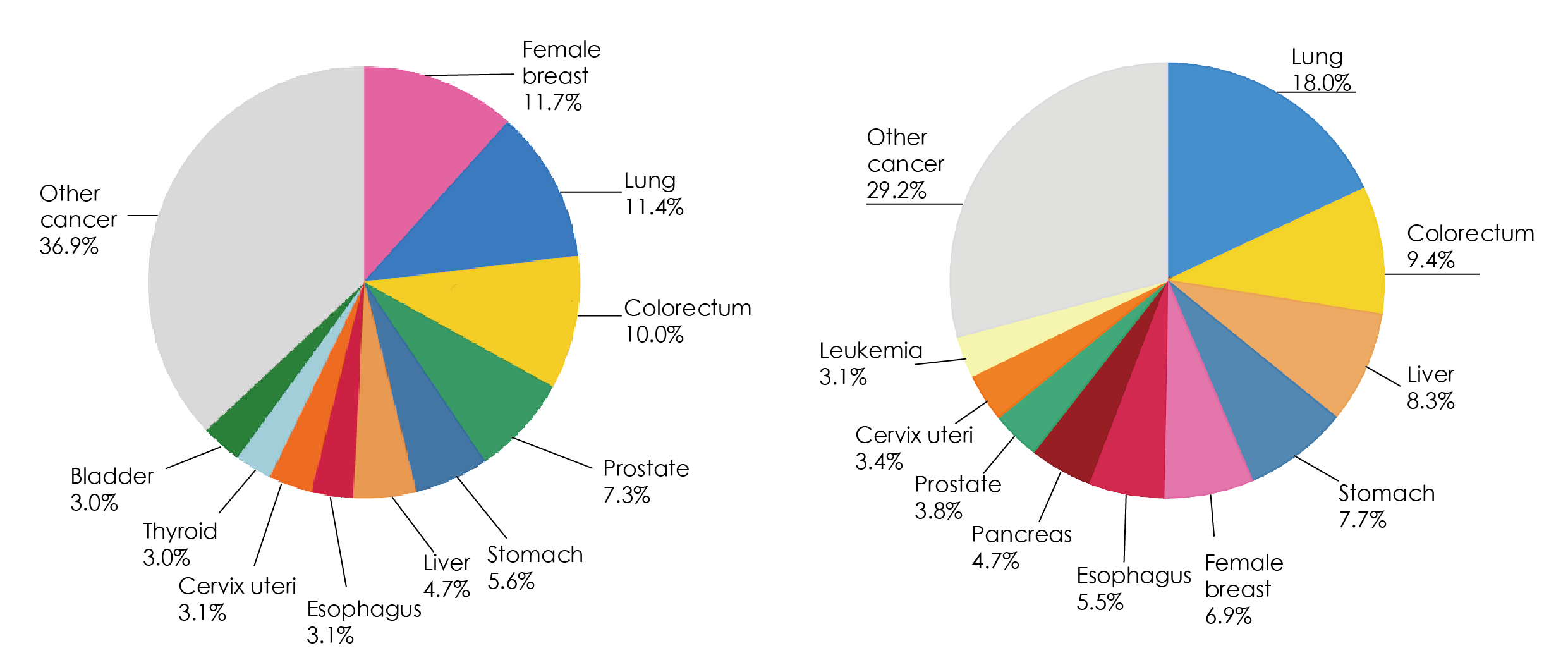
World Health Organization International Agency for research on cancer (IARC) 2020 global cancer data: in 2020, there were 1.93 million cases of colorectal cancer worldwide.
Application of molecular detection in colorectal cancer:
With the development of deep research and detection level of tumor related molecular markers such as Ras, BRAF, mismatch repair / microsatellite instability (MMR / MSI), reasonable detection technology and application have become an important part of clinical practice.
PRODUCT INFORMATION
| Product Name | Core Technology | Pack Size | Instruments Validated | Sample Type |
Genecan® Colorectal Cancer Related Gene Mutation Detection Kit | RingCap® | 16 Tests/Kit 32 Tests/Kit | Illumina MGISEQ | Tumor tissue, Ascites |
| Targeted Drugs and Immunotherapy Related Genes | Prognosis Related Genes | Chemosensitivity Gene |
| KRAS, NRAS, BRAF, PIK3CA, ERBB2, MSI | TP53 | UGT1A1,DYPD |
APPLICABLE PEOPLE
1. Recommendation on stage I ~ III colorectal cancer: before adjuvant treatment decision of colorectal cancer.
2. Recommendation on stage IV colorectal cancer: before the decision of first-line treatment plan.
3. Formulate diagnosis and treatment strategies and schemes according to different molecular characteristics of colorectal cancer.
DETECTION SIGNIFICANCE
1. It contains RAS, BRAF and HER2 genes to predict the response to anti-EGFR monoclonal antibody, BRAF/MEK inhibitor or anti-HER2 therapy.
2. It contains marker MSI site of immunosuppressant Pembrolizumab.
3. It contains UGT1A1 and DYPD genes related to chemotherapy, which can adjust the medication of irinotecan, fluorouracil, capecitabine and Ftorafur.The level of evidence for the effectiveness of chemotherapy genes can refer to PharmGKB database .
4. It includes TP53 tumor suppressor gene detection, which provides reference for patient prognosis evaluation and inclusion in AZD1775 clinical trial .
5. The MSI site adopts single nucleotide site, which effectively avoids the problem of polynucleotide polymorphism and can meet the detection of single sample MSI .
FEATURES & ADVANTAGES
1.Ease of Use: Based on the independent patent technology RingCap®, library preparation in 2 steps.
2.Fast Results: The library preparation takes only 3.5 hours, 2 working days from sampling to reporting.
3.High Sensitivity: The sensitivity tissue can reach up to 1%, blood up to 0.5%.
4.Comprehensive Coverage: Covers and detect 6 genes related to targeted drugs and immunosuppressants, 34 MSI loci and 2 chemotherapy related genes.
Detection Process
1.Nucleic Acid Extraction
2.Library Preparation (3.5 hours total time)
3.Sequencing
4.Auto-data Analysis
5.Report