Current location: Home > NEWS > Industry news
NEWS
PRODUCTS
The Things Between Thyroid Cancer and the TERT Gene
News source: Release time:[2024-03-22]
In recent years, the incidence of thyroid cancer has been continuously and rapidly rising in many countries and regions worldwide. According to surveys, there are over 300 million people globally suffering from thyroid diseases, among which female patients outnumber male patients by eight times, with a prevalence rate of over 10% among women over 40 years old.[1]
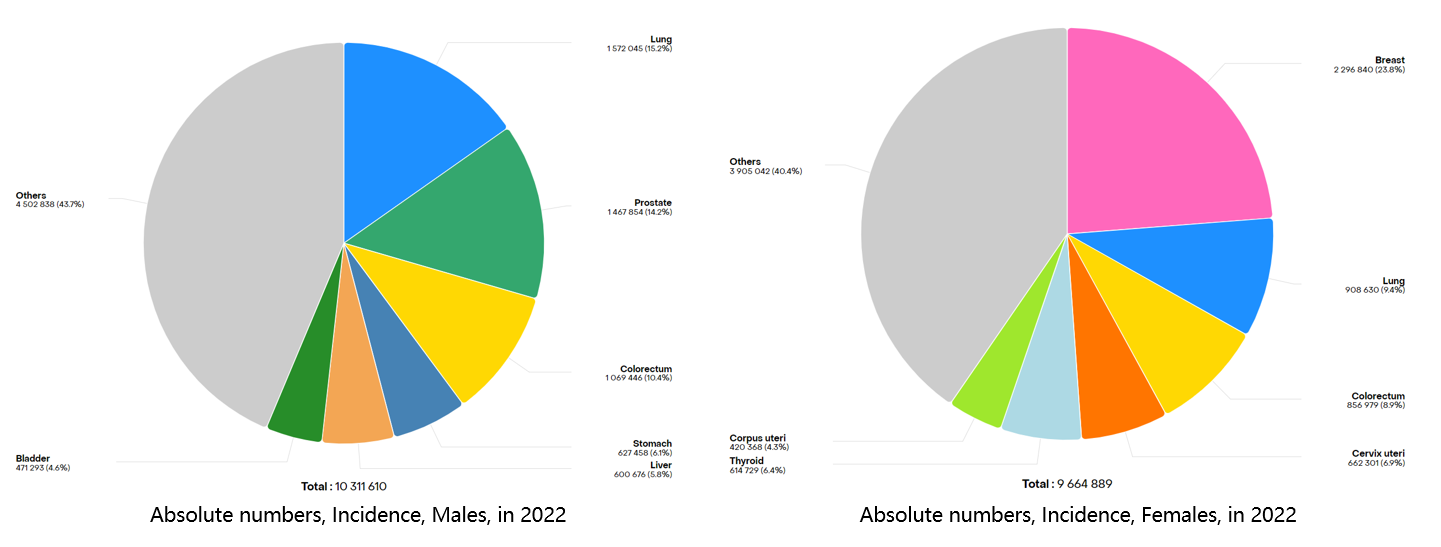
Figure 1 Top ten cancers with new cancer cases worldwide in 2022
Thyroid cancer is the most common malignant endocrine tumor. According to the origin and differentiation of the tumor, it can be divided into differentiated thyroid cancer (DTC), medullary thyroid cancer (MTC), poorly differentiated thyroid cancer (PDTC), and undifferentiated thyroid cancer (ATC). Among them, DTC is further divided into papillary thyroid cancer (PTC) and follicular thyroid cancer (FTC).[2] The lower the degree of tumor differentiation, the higher its invasiveness, and the worse the prognosis.
Different subtypes of thyroid cancer have different genetic molecular characteristics. For example, PTC commonly exhibits BRAF gene mutations (see "What You Need to Know About Thyroid Cancer and the BRAF Gene"); MTC commonly presents RET gene mutations (see "What You Need to Know About Thyroid Cancer and the RET Gene"); FTC commonly shows PAX8-PPARγ fusion or RAS mutations. In PDTC and ATC, common gene mutations include TP53, TERT, CDKN2A-RB1, and PTEN.[3-6] Each mutated gene in these subtypes leads to loss of control of the cell cycle, resulting in carcinogenesis. However, among these mutations, TERT gene mutation can be said to reinforce this loss of control by eliminating intrinsic aging barriers, thereby granting cells indefinite lifespan. Research indicates that the incidence of TERT gene mutations is 10% to 15% in DTC, 40% to 45% in PDTC and ATC, while being rare in benign nodules.[3] Therefore, the TERT gene is an important tumor marker for the diagnosis and assessment of aggressiveness in thyroid cancer.
The TERT gene is located on chromosome 5p15.33, containing 16 exons and 15 introns, encoding telomerase reverse transcriptase, which plays a crucial role in maintaining telomere stability, genome integrity, long-term cellular activity, and the unlimited proliferative capacity of tumor cells.[7] It forms telomerase together with the telomerase RNA component (TERC), crucial for maintaining telomere length and determining telomerase activity. In normal cells, telomerase activity is typically tightly regulated, with low levels in most tissues, only present and highly regulated in a few cells such as germ cells and hematopoietic stem cells. However, in tumor cells, over 90% exhibit overexpression of telomerase, allowing for maintenance or elongation of telomeres, leading to malignant proliferation and immortality.[8] Activation of TERT is primarily due to mutations occurring in the promoter region, with the most common being the C228T point mutation upstream of the TERT transcription start site and the C250T point mutation. In thyroid cancer, the C228T mutation is more common than C250T, and the two are mutually exclusive[9]. TERT promoter mutations can increase TERT transcription levels, but expression is regulated by ETS transcription factors, with the most closely related being GA binding protein A (GABPA) and GA binding protein B (GABPB). Studies have shown that TERT promoter mutations generate new ETS transcription factor binding sites, where the GABPA-GABPB complex typically binds to ETS transcription factor binding sites as a heterotetramer, thereby promoting TERT transcription and telomerase activation, and facilitating tumor initiation and progression.[10] ETS transcription factors, especially GABPA or GABPB1, have been proposed as new targets for cancer therapy.
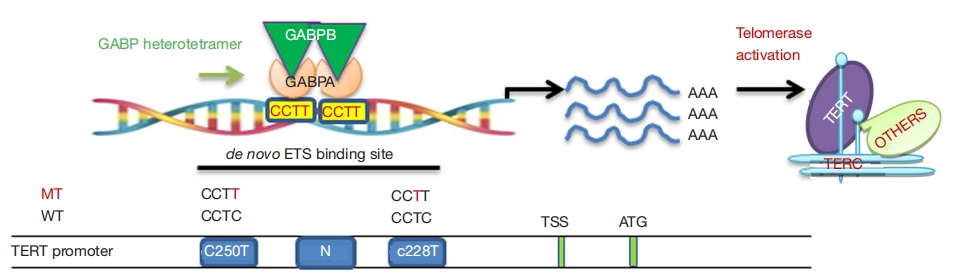
Figure 2 The carcinogenic mechanism of TERT gene
01. TERT Promoter Mutations and Prognosis in Thyroid Cancer
A study compared the overall survival and disease-free survival of 93 patients with papillary thyroid carcinoma (PTC) who had either wild-type (wt) or mutant-type (mt) TERT promoter. The results showed a significantly shortened overall survival and disease-free survival in mt TERT patients. This finding was further validated by a study from the TCGA cohort. Numerous clinical studies have demonstrated a strong association between TERT promoter mutations and advanced or progressive disease, significantly impacting the prognosis or mortality rate of thyroid cancer patients [11].
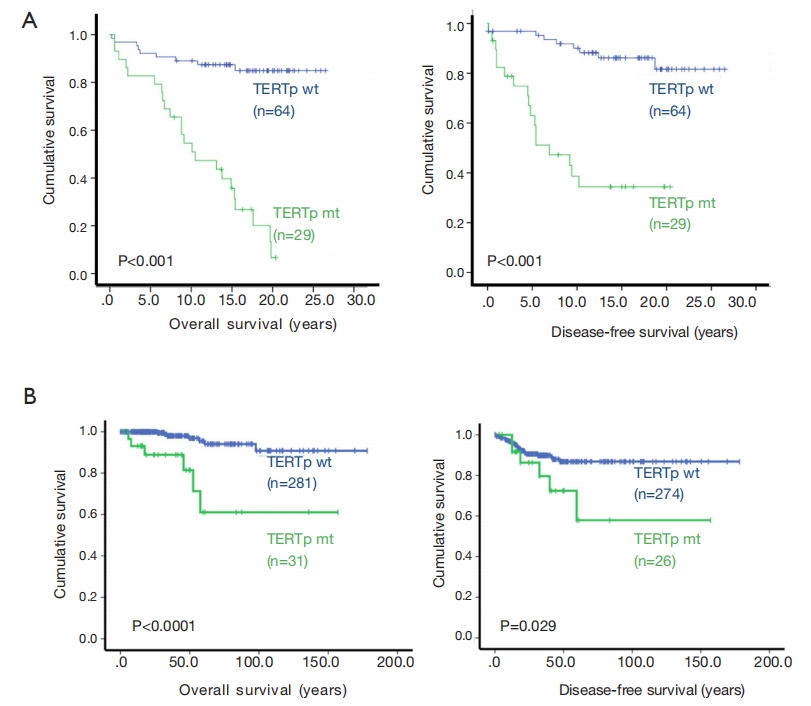
Figure 3 TERT gene promoter mutation and prognosis
02. Synergistic Effect of TERT and BRAF V600E Mutation
Liu et al. found that the transcription factor FOS can be phosphorylated and activated by the BRAF V600E-mediated MAPK signaling pathway, and it binds with GABPB to increase its expression. This leads to an increase in the formation of the GABPA-GABPB complex, which binds to the mutated TERT promoter, activating TERT. This also explains why mutations in the TERT promoter frequently coexist with BRAF V600E mutations in PTC, and the presence of these two mutations is associated with increased invasiveness, lymph node and distant metastases, tumor recurrence, and mortality rates [12].
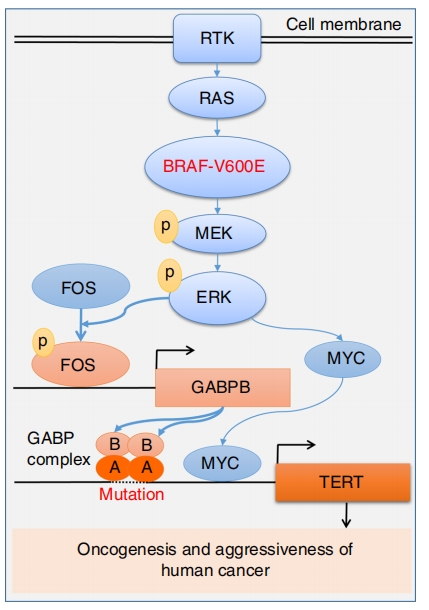
Figure 4 Oncogenic cooperation between TERT promoter and BRAF V600E mutation
03. TERT Promoter Mutation and Treatment
Radioactive 131I is an important adjuvant therapy for thyroid cancer, which can reduce recurrence and prolong survival. However, radioiodine resistance is also a phenomenon that may be encountered clinically, indicating poor response of patients to radioactive isotope therapy. Numerous studies have found a significant correlation between the presence of TERT promoter mutations and reduced 131I uptake or iodine resistance. Therefore, TERT promoter mutations can serve as early predictive factors for radioiodine resistance. When TERT promoter mutations are combined with BRAF V600E double mutations, it suggests insensitivity to radioiodine and poor efficacy of isotope therapy, while studies have shown that combined treatment with BRAF V600E and MEK inhibitors has better efficacy, and preventive neck lymph node dissection may be performed to improve cure rates.[14-15] Due to TERT promoter mutations leading to telomerase activation, which in turn leads to the occurrence and development of thyroid cancer, inhibiting telomerase activity is also a potential treatment option. Imetelstat (GRN163L), a telomerase inhibitor, has been shown to effectively inhibit telomerase activity in various malignant cells and is the only telomerase inhibitor included in clinical trials[16]. GRN163L has been proven to be very effective in the treatment of myeloproliferative tumors, and its application in the treatment of thyroid cancer with GRN163L is also likely to be an effective treatment method, even exploring combined treatment with BRAF inhibitors is a promising treatment option[17].
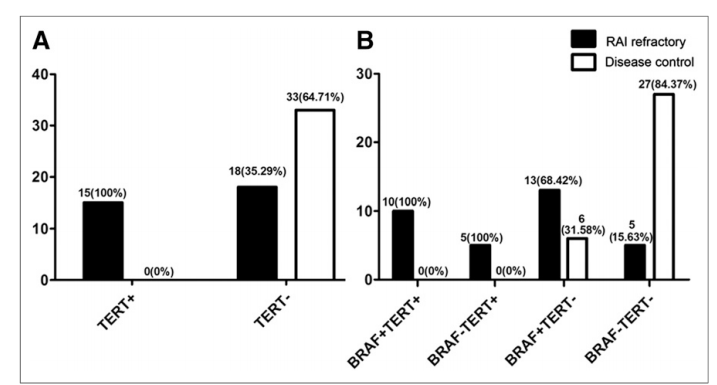
Figure 5 Relationship between TERT/BRAF mutations and response to radioactive iodine therapy
TERT plays a crucial role in tumor progression and the worsening of prognosis. The potential clinical applications of TERT expression and telomerase activation or inhibition of telomerase activity remain at the forefront of many cancer treatment strategies. TERT promoter mutations serve as specific markers for the diagnosis and prognosis of thyroid cancer. When combined with BRAF V600E mutation or other genetic markers (such as RAS mutations), they can help avoid misdiagnosis of thyroid cancer, better assess patient prognosis, and suggest treatment options.
References:
[1] IARC Global Cancer Burden Data 2022.
[2] Thyroid Cancer Diagnosis and Treatment Guidelines (2022 edition).
[3] Expert Consensus on Genetic Testing and Clinical Applications of Thyroid Cancer in Guangdong (2020 edition).
[4] Expert Consensus on Thyroid Cancer RET Gene Testing and Clinical Applications (2021 edition).
[5] Expert Consensus on Diagnosis and Treatment of Thyroid Undifferentiated Carcinoma (2023 edition).
[6] J Clin Invest 2016,126:1052-66.
[7] Science 2013,339,957-959.
[8] Life Sci,2020,257:118115.
[9] Endocr Relat Cancer. (2016) 23:R143-55.
[10] Blood Cells Mol Dis 2004;32:143-54.
[11] Annals of Translational Medicine, 2020, 8(19).
[12] Nat Commun 2018;9:579.
[13] J Nucl Med 2017,58:258-65.
[14] Endocrinol Metab Clin North Am, 2019,48(1):109-124.
[15] Proc Natl Acad Sci U S A 2020,117:15846-51.
[16] Curr Top Med Chem 2020,20:410-32.
[17] Biochem Biophys Res Commun 2020,527:425-31.
Disclaimer: This article is for sharing purposes only. If there are any copyright issues, please contact us as soon as possible, and we will correct them promptly. Thank you!