Current location: Home > NEWS > Industry news
NEWS
PRODUCTS
Is Gut Microbiota an Accomplice in Cancer?
News source: Release time:[2024-03-12]
Gut Microbiota
Gut microbiota, the microorganisms residing in the human intestines, have silently influenced our health for a long time. They are closely related to our physiological functions, and some even consider them as another "organ" within the human body. However, their role is far more complex than we imagine. In recent years, research on the relationship between gut microbiota and cancer has deepened continuously, attracting significant attention. Are these microorganisms enemies or accomplices of cancer? Before delving into this question, let's first review some basic knowledge about gut microbiota. Gut microbiota is a complex ecosystem composed of various microorganisms, which influence our digestion, immune system, and other systems through interactions with the intestines. Due to this close relationship, gut microbiota also has profound effects on the occurrence and development of cancer.
However, did you know that there are significant differences in cancer incidence and mortality rates among different races and ethnicities? An opinion review article published in Nature Reviews Cancer discusses how differences in gut microbiota resulting from social, community, and individual factors such as diet, environment, lifestyle habits, and policies under structural racism affect the racial and ethnic disparities in cancer [1].
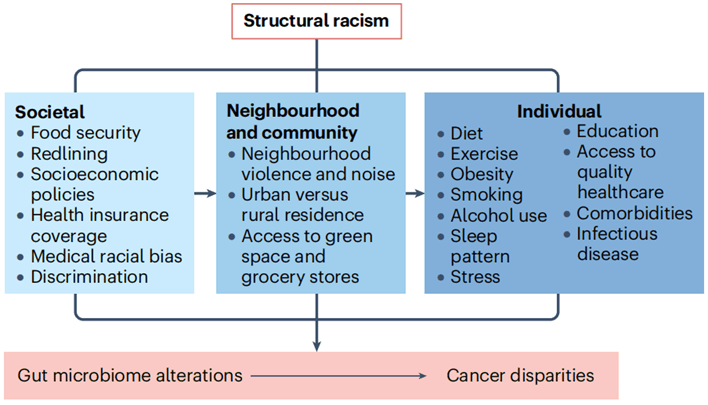
Figure 1 Multi-level exposures that impact gut microbiota and subsequently contribute to cancer disparities
Whether there is a causal relationship between the gut microbiota and cancer, Xiangya Hospital's Zhu Hongli used publicly available databases to conduct Mendelian randomization (MR) calculations on two samples[2], exploring and confirming a certain causal relationship between the gut microbiota and eight types of cancer. For example, there are 11 causal relationships between genetic susceptibility of the gut microbiota and cancer, including the genus Bifidobacterium; there are 17 strong correlations between genetic susceptibility of the gut microbiota and cancer, such as the family Bifidobacteriaceae and the order Bifidobacteriales being risk factors for breast cancer, while the genus UCG013 is a protective factor against breast cancer. This study helps provide new insights into the further mechanisms and clinical research of microbiota-mediated cancers.
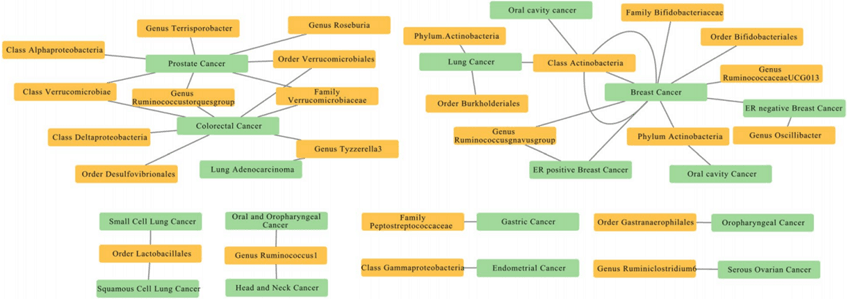
Figure 2 Mendelian random analysis of the causal relationship between intestinal flora and cancer
The incidence of colorectal cancer (CRC) is on the rise, making it the second leading cause of cancer-related deaths globally, emphasizing the urgent need for improved prevention and treatment strategies. The intestinal microbiota, consisting of trillions of microorganisms, has been closely linked to the occurrence and progression of CRC. A recent review article published in Nature Reviews Microbiology [3] summarized the research evidence regarding the overall disruption of intestinal microbiota and the association of specific microorganisms with CRC in patients. It elucidated the potential mechanisms by which microbes drive the occurrence of CRC and the possible role of complex microbial communities in the pathogenesis of CRC. The review also discussed the clinical prospects of microbiota-targeted strategies for the prevention and treatment of CRC.
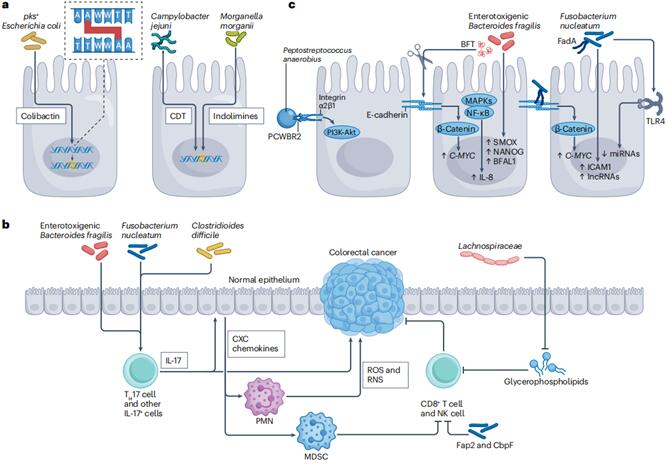
Figure 3 Microbial mechanisms of colorectal cancer
In the study of colorectal cancer, the relationship between the gut microbiota and cancer is particularly intriguing. Specific gut bacteria have been found to be associated with an increased risk of colorectal cancer, while others may have protective effects. These findings provide new directions for the future prevention and early diagnosis of cancer. In the causation of colorectal cancer by the gut microbiota, the metabolites produced by the gut microbiota play a crucial role. They can reshape the tumor microenvironment, regulate key signaling pathways in cancer cells and various immune cells, affecting the occurrence and development of cancer as well as the effectiveness of cancer treatment. Meng Xuli's team at Zhejiang Provincial People's Hospital recently published a comprehensive review in Advanced Science, detailing the mechanisms by which gut microbiota metabolites affect cancer progression and their roles in radiotherapy, chemotherapy, and immunotherapy[4].
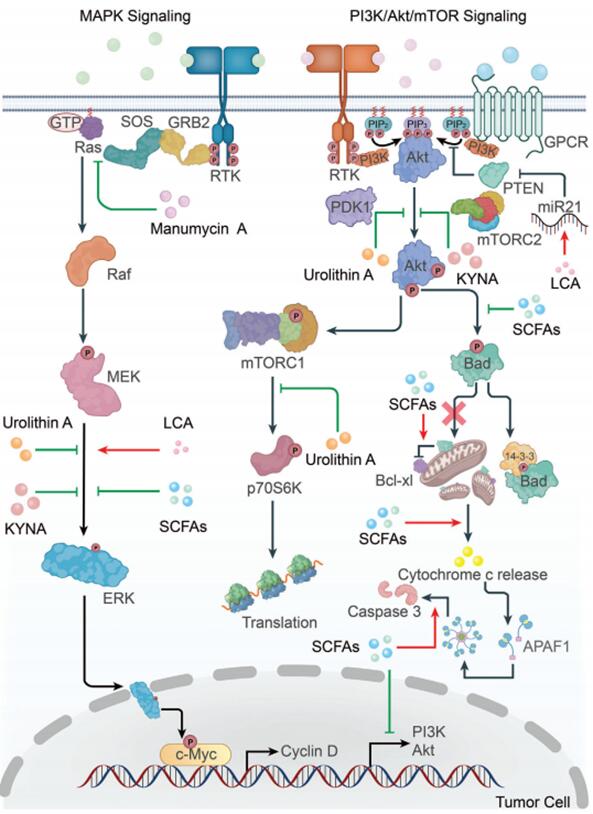
Figure 4 Microbial metabolites participate in tumor cell signal regulation and tumor progression
Microbial metabolites serve as a crucial link between gut bacteria and cancer, regulating cancer progression by reshaping the tumor microenvironment, influencing immune cell function and cytokine levels. These metabolites modulate signaling pathways such as MAPK, PI3K/Akt, and Wnt, affecting tumor cell proliferation, apoptosis, and metastasis, as well as mitigating side effects and enhancing the efficacy of radiation/chemotherapy. Apart from traditional strategies involving antibiotics to disrupt the microbiota, new approaches based on microbial metabolites, including diet, drug design, fecal microbiota transplantation, and probiotics, have been developed. In treatment, it's essential to consider the patient's condition comprehensively to avoid conflicts between metabolites, microbiota, and anticancer drugs, which could render the treatment ineffective or exacerbate side effects. This highlights the potential for better application of microbial metabolites in cancer treatment and the development of new therapeutic strategies based on these metabolites, deserving attention.
In treatment, the microbiome also plays a crucial role in cancer therapy. For example, the efficacy of immune checkpoint inhibitors is influenced by both intestinal and tumor microbiota. Additionally, metabolites produced by gut bacteria also play an important role in tumor progression and cancer treatment. These findings provide new insights and potential therapeutic targets for future cancer treatment.
Over the past decade, cancer treatment has undergone a revolution from traditional therapies to immune checkpoint inhibitors (ICI). These immune therapies can stimulate the host immune system to fight against tumors and have achieved unprecedented long-term remission effects. A review article in Trends in Molecular Medicine explores the unexpected role of bacteria in reshaping tumor immune responses. Research on symbiotic communities and tumor microbiomes is expected to identify which patients can benefit from immune therapy and help expand the scope of ICI treatment, improving treatment outcomes[5].
Research has found that cancer patients with high gut microbiota diversity respond better to immune checkpoint inhibitor (ICI) therapy; gut and tumor microbiota can directly downregulate the expression levels of immune checkpoint molecules on immune cells and mobilize anti-tumor immune cells by producing metabolites such as indole, adenosine, and short-chain fatty acids; gut microbiota can reduce ICI-related side effects such as colitis; dysbiosis may lead to excessive production of inflammatory factors, exacerbating the side effects of ICI therapy; clinically, dietary interventions, prebiotics, probiotics, fecal microbiota transplantation, and antibiotics can be used to improve gut microbiota composition and ICI efficacy.
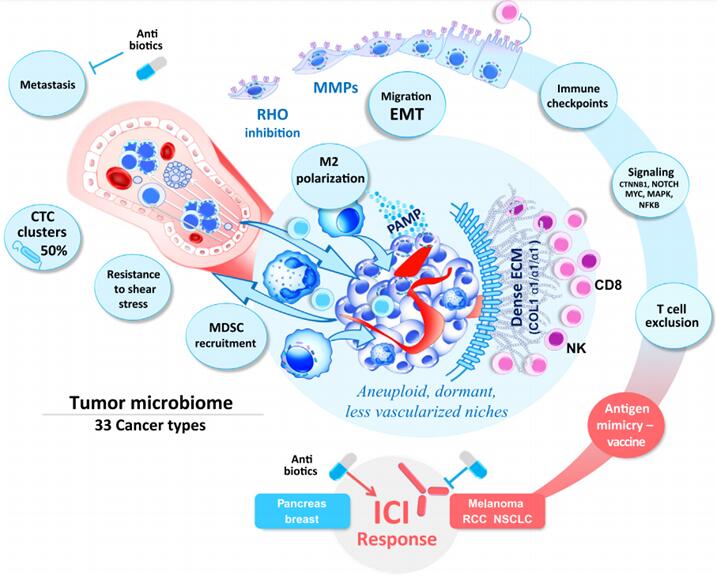
Figure 5 The driving role of intratumoral bacteria in tumor progression
The relationship between cancer and the gut microbiota is a complex and multidimensional topic. Increasing evidence suggests that the microbiome plays a crucial role in the development and treatment of cancer. However, whether the microbiota acts as an enemy or an accomplice in cancer remains a specific issue that requires detailed analysis.
Overall, the gut microbiota plays an undeniable role in the development and treatment of cancer. While further exploration of its specific mechanisms and potential therapeutic strategies is needed, these research findings undoubtedly pave the way for new directions in future cancer research. In future studies, a deeper understanding of the interaction between the gut microbiota and cancer is necessary to provide more effective personalized treatment plans for patients.
References:
[1] Byrd D, Wolf P. The microbiome as a determinant of racial and ethnic cancer disparities. Nat Rev Cancer. 2023 Oct 23.
[2] Long Y, Tang L, Zhou Y, Zhao S, Zhu H. Causal relationship between gut microbiota and cancers: a two-sample Mendelian randomisation study. BMC Med. 2023 Feb 21;21(1):66.
[3] White MT, Sears CL. The microbial landscape of colorectal cancer. Nat Rev Microbiol. 2023 Oct 4.
[4] Yang Q, Wang B, Zheng Q, Li H, Meng X, Zhou F, Zhang L. A Review of Gut Microbiota-Derived Metabolites in Tumor Progression and Cancer Therapy. Adv Sci (Weinh). 2023 May;10(15):e2207366.
[5] Guillot N, Roméo B, Manesh SS, Milano G, Brest P, Zitvogel L, Hofman P, Mograbi B. Manipulating the gut and tumor microbiota for immune checkpoint inhibitor therapy: from dream to reality. Trends Mol Med. 2023 Nov;29(11):897-911.
Note: This text is for sharing purposes only. If there are any copyright issues, please contact us as soon as possible, and we will correct it promptly. Thank you!