Current location: Home > NEWS > Industry news
NEWS
PRODUCTS
One article to understand the progress in the treatment of early stage lung cancer - targeted therapy VS immunotherapy
News source: Release time:[2024-02-29]
In clinical practice, the most common treatment for early-stage NSCLC (Non-Small Cell Lung Cancer) is surgical treatment.[1] However, the needs for adjuvant and neoadjuvant therapy in this population have not been fully met.[2] Given the significant risk of postoperative recurrence and death, there is an urgent need to improve the treatment outcomes for resectable NSCLC. Traditional chemotherapy has shown limited benefits in adjuvant and neoadjuvant therapy for NSCLC, with only a respective increase of 5.4% and 5% in the 5-year survival rate. [3-4]In recent years, the rapid development of targeted therapy and immunotherapy has greatly improved the survival outcomes for patients with advanced NSCLC. Based on this progress, their application value in early-stage NSCLC patients has become a hot exploration direction.
Targeted Therapy
01
EGFR Mutation-Driven Adjuvant Targeted Therapy for Resectable Non-Small Cell Lung Cancer
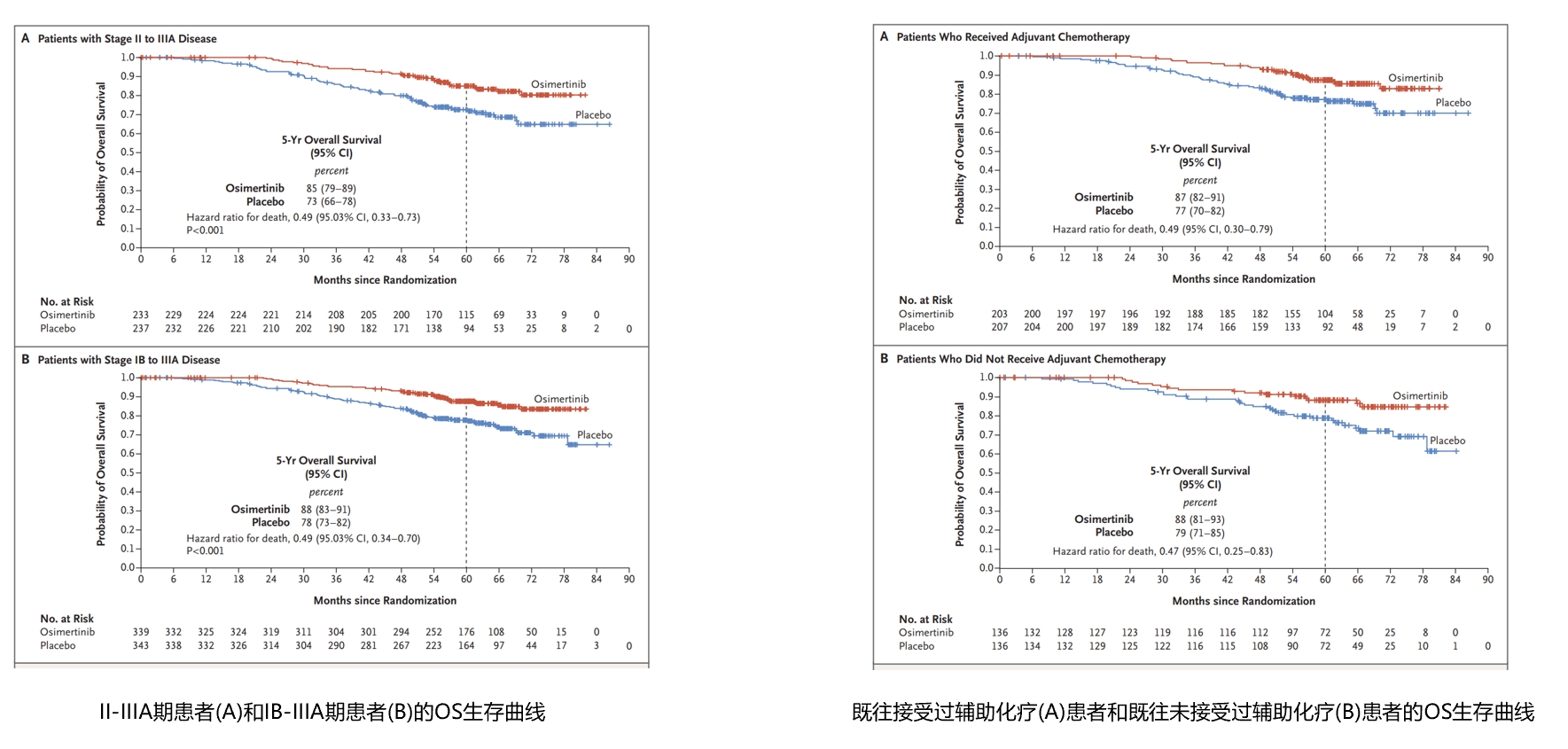
In 2020, the ADAURA study announced the achievement of the primary endpoint of Disease-Free Survival (DFS). Osimertinib adjuvant therapy was approved by the US Food and Drug Administration (FDA) in December 2020 and by the National Medical Products Administration (NMPA) in April 2021. In 2023, the ADAURA study demonstrated successful transformation of DFS benefit into Overall Survival (OS) benefit and was published in the New England Journal of Medicine (NEJM). In patients with stage II-IIIA disease, the median follow-up times for the osimertinib group and the placebo group were 59.9 months and 56.2 months, respectively, with 5-year OS rates of 85% and 73%, respectively, and a hazard ratio (HR) of 0.49 (95.03% CI, 0.33-0.73, P<0.001).In patients with stage II-IIIA disease, the median follow-up times for the osimertinib group and the placebo group were 60.4 months and 59.4 months, respectively, with 5-year OS rates of 88% and 78%, respectively, and a hazard ratio (HR) of 0.49 (95.03% CI, 0.34-0.70, P<0.001). [5] Subgroup analysis also showed benefits regardless of prior adjuvant chemotherapy (OS HRs were 0.49 and 0.47, respectively). This further confirms that osimertinib as postoperative adjuvant therapy is well-deserved as the "gold standard" for the treatment of EGFR mutation-positive NSCLC patients.
02
ALK-positive operable non-small cell lung cancer adjuvant targeted therapy[6]
ALINA is an international multicenter, open-label, randomized, phase III clinical trial designed to explore the efficacy and safety of alectinib compared to platinum-based chemotherapy as adjuvant therapy in early-stage ALK-positive NSCLC patients. It is also the world's first and currently only phase III clinical study to demonstrate disease-free survival (DFS) benefit in ALK-positive early-stage non-small cell lung cancer (NSCLC) patients receiving postoperative adjuvant therapy.
In the II-IIIA population, the median follow-up for the alectinib group was 27.9 months (vs. 27.8 months for the chemotherapy group), with a significant DFS benefit observed in the alectinib group compared to the chemotherapy group (HR 0.24; 95% CI 0.13-0.45; P<0.0001). In the ITT population, the median follow-up for alectinib was 27.8 months (vs. 28.4 months for the chemotherapy group), and again a DFS benefit was observed in the alectinib group (HR 0.24; 95% CI 0.13-0.43; P<0.0001), indicating a 76% reduction in the risk of recurrence or death with alectinib adjuvant therapy.
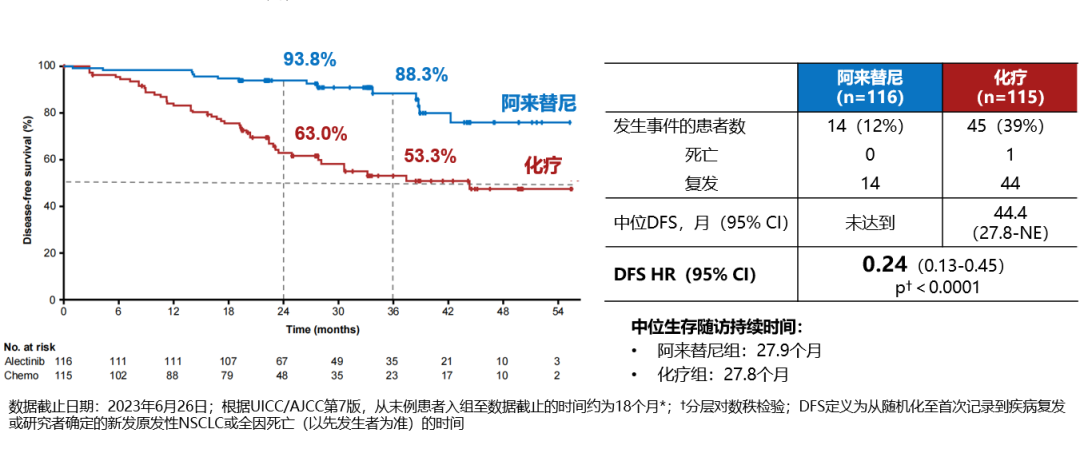
DFS K-M curve for stage II-IIIA patients
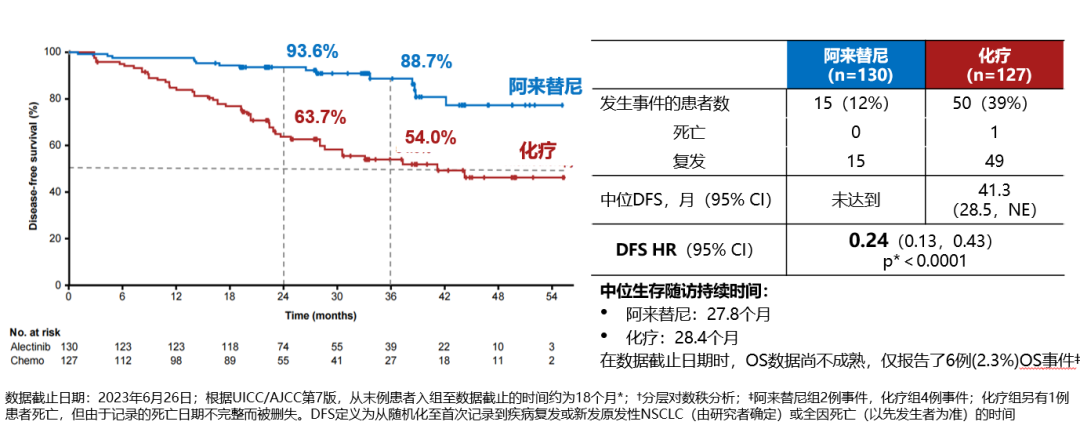
DFS K-M curve for ITT population (stage IB-IIIA patients)
Immune therapy
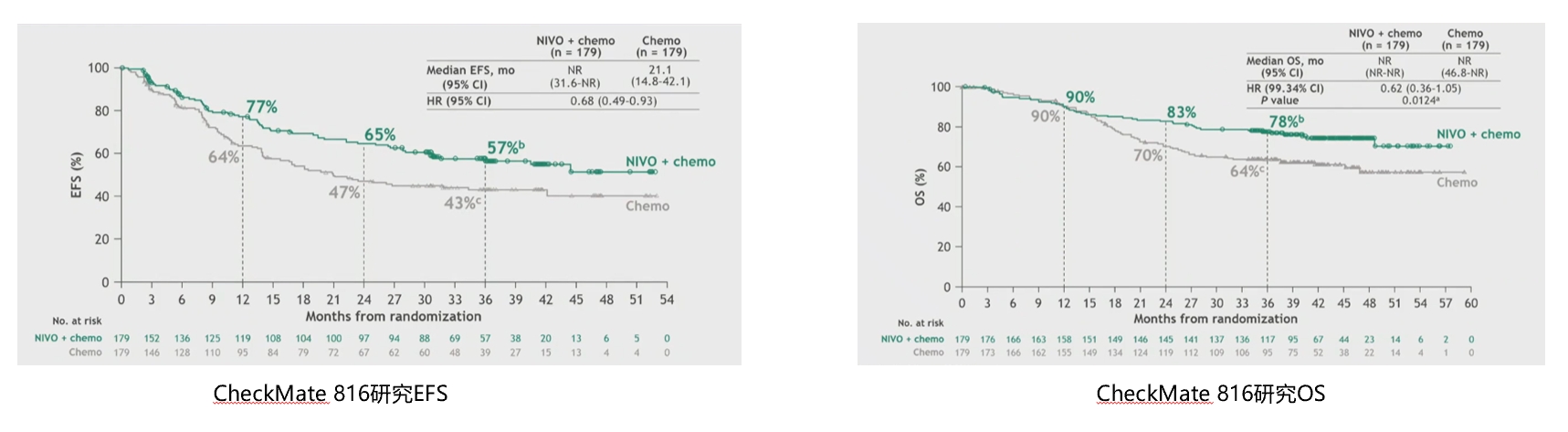
CheckMate-816 is the first Phase III clinical trial of immune neoadjuvant therapy, including 358 patients with IB (≥4cm) to ⅢA stage NSCLC. At the 2021 American Association for Cancer Research (AACR) annual meeting, data on one of the main endpoints of the study, pathological complete response rate (pCR), were announced. The results showed that compared to neoadjuvant chemotherapy alone, neoadjuvant nivolumab plus chemotherapy significantly improved the pCR rate in patients (24.0% vs. 2.2%, P <0.0001). [7]At the 2022 AACR annual meeting, CheckMate-816 announced the EFS results: compared to chemotherapy alone, nivolumab plus chemotherapy significantly improved patient EFS (median EFS: 31.6 months vs. 20.8 months, HR=0.63, p=0.0052). In the 2023 ELCC meeting, the 3-year follow-up of the CheckMate-816 study was updated: the median EFS in the nivolumab plus chemotherapy experimental group still had not been reached, while it was 21.1 months in the chemotherapy control group (HR=0.68), with 3-year EFS rates of 57% and 43% for nivolumab plus chemotherapy and chemotherapy alone groups, respectively. With prolonged follow-up time, the OS curves of the nivolumab plus chemotherapy group and the chemotherapy alone group became increasingly separated, and the median OS for both groups had not yet been reached (HR=0.62, p=0.0124), with a 14% increase in 3-year OS rate for the nivolumab plus chemotherapy group.[8]
KEYNOTE-671 is currently the only early NSCLC study with statistical significance, with OS and EFS as dual primary endpoints, achieving positive results in both endpoints. After a median follow-up of 36.6 months, pembrolizumab combined with chemotherapy as neoadjuvant therapy, followed by adjuvant monotherapy, significantly improves patient OS: compared to the control group, regardless of PD-L1 expression, patients in the pembrolizumab treatment group had a 28% reduction in the risk of death (HR=0.72, 95% CI, P=0.00517), and the median OS in the pembrolizumab group has not yet been reached, with a 3-year OS rate of 71.3%, compared to 52.4 months and 64.0% in the control group, respectively. Compared to placebo ± chemotherapy, the pembrolizumab group showed an increase in mEFS of nearly 2 and a half years (47.2 months vs. 18.3 months, HR=0.59). The 3-year EFS rates in the two groups were 54.3% and 35.4% [9].
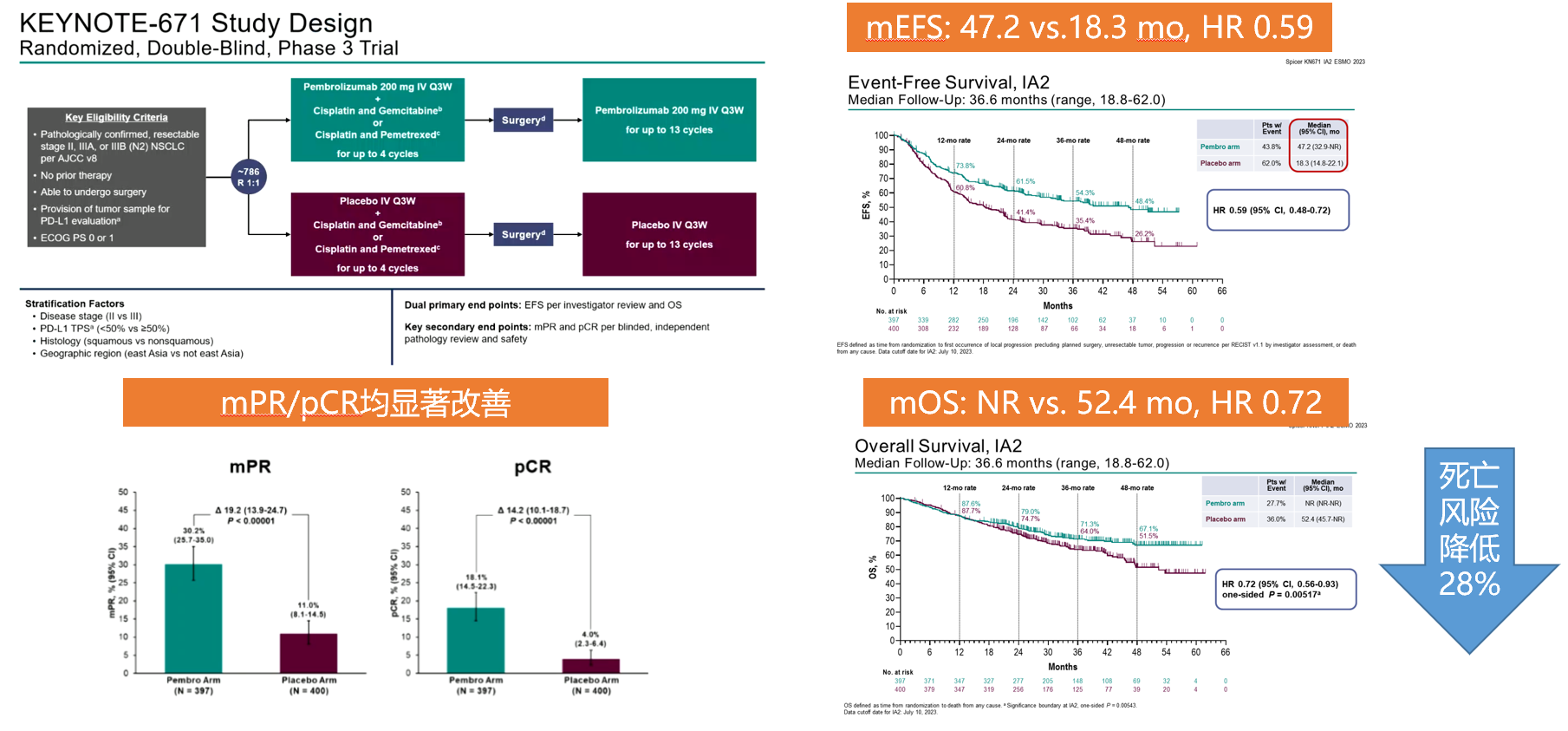
KEYNOTE-671 Study Data
From the perspective of clinical studies combined with adjuvant immunotherapy, early initiation of immunotherapy for resectable NSCLC patients, followed by continued immunotherapy postoperatively, may potentially yield greater benefits for patients. Additionally, from the standpoint of pathological response, patients achieving pCR demonstrate superior prognostic outcomes.
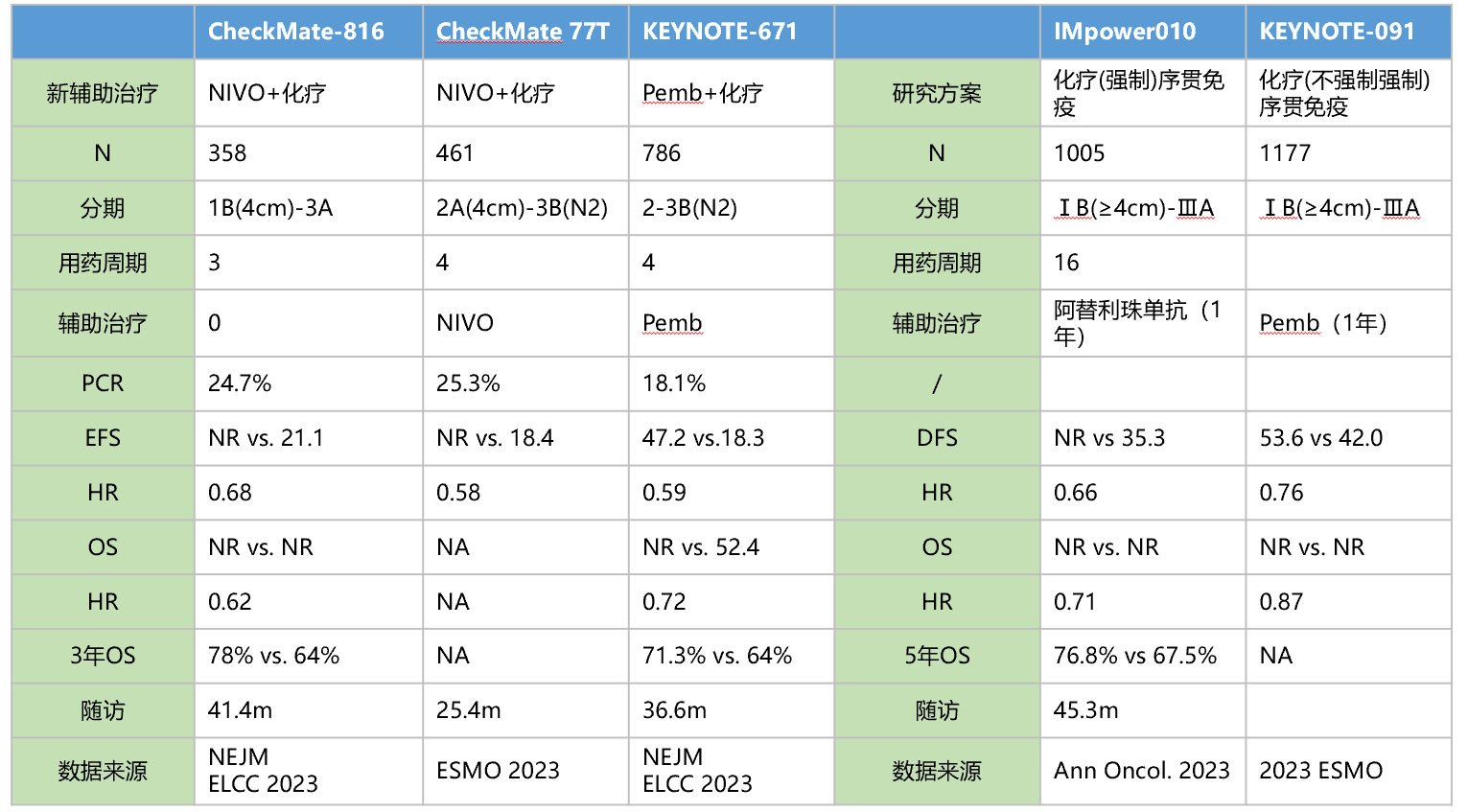
Summary of perioperative immunotherapy research
Summary
Postoperative adjuvant therapy is an important treatment modality for reducing recurrence, prolonging survival, and improving quality of life in patients with early to mid-stage NSCLC tumors after complete resection. From classic postoperative adjuvant chemotherapy to EGFR-TKI adjuvant targeted therapy established by studies such as ADAURA and ADJUVANT, adjuvant therapy has brought increasing clinical benefits to early to mid-stage NSCLC patients. According to current evidence, EGFR gene mutation testing is recommended for early to mid-stage NSCLC patients, and adjuvant therapy strategies should be formulated based on EGFR mutation status and tumor staging.
Immunotherapy has been applied to perioperative lung cancer patients, with several studies yielding positive results and corresponding indications approved. However, many questions remain unanswered, such as which NSCLC patients can benefit from perioperative immunotherapy, whether PD-L1 expression level is the best or only benefit indicator, all of which are currently unknown and require further exploration.
Targeted and immunotherapy applied to early-stage lung cancer patients have brought tangible benefits, but it's important to recognize that there are still many issues in clinical practice that warrant further exploration. We look forward to current and future research efforts to support further optimization of treatment strategies, bringing more clinical benefits to early to mid-stage resectable NSCLC patients, and achieving patient cure.
References
[1] 2023 WCLC.P1.25-06. Real-world Primary Treatment Patterns in Stages I-III Non-Small Cell Lung Cancer P1.25.
[2] 2023 WCLC.WS02.07. Beyond Checkmate 816: What Is the Future in Perioperative Immune-Oncology (IO).
[3] Pignon JP, Tribodet H, Scagliotti GV, et al. J Clin Oncol. 2008 Jul 20;26(21):3552-9.
[4] NSCLC Meta-analysis Collaborative Group. Lancet. 2014 May 3;383(9928):1561-71.
[5] N Engl J Med 2023; 389:137-147
[6] Benjamin J. Solomon, et al. Efficacy and safety of adjuvant alectinib versus chemotherapy in patients with early-stage ALK+ non-small cell lung cancer (NSCLC). 2023 ESMO. LBA2.
[7] N Engl J Med. 2022 Aug 11;387(6):571.
[8] P.M. Forde, J. Spicer, N. Girard, et al. Neoadjuvant nivolumab (N) + platinum-doublet chemotherapy (C) for resectable NSCLC: 3-y update from CheckMate 816. 2023 ELCC. Abs 84O.
[9] Spicer JD, et al. Overall survival in the KEYNOTE-671 study of perioperative pembrolizumab for early-stage non-small-cell lung cancer (NSCLC).2023 ESMO, LBA56.
Declaration: This article is for sharing only. If there are any copyright issues, please contact us as soon as possible, and we will correct them promptly. Thank you!