Current location: Home > NEWS > Industry news
NEWS
PRODUCTS
Brief Summary of Neoadjuvant Immunotherapy Research Progress in NSCLC
News source: Release time:[2023-04-27]
Background Introduction
As the most common cancer with the highest incidence and mortality rates worldwide, lung cancer has attracted significant attention from people worldwide. Non-small cell lung cancer (NSCLC), the most common type of lung cancer, accounts for 80%-85% of all lung cancers. Due to the subtle early clinical manifestations of NSCLC, clinical data shows that 75% of NSCLC patients are already at an advanced stage when diagnosed. For NSCLC patients at an advanced stage, surgical treatment remains the preferred method. However, approximately 25%-35% of patients still experience recurrence and metastasis after complete tumor resection, resulting in a lower five-year survival rate [1]. Therefore, systemic treatment should be administered as early as possible to eliminate or avoid micro-metastatic lesions to the maximum extent. In recent decades, significant progress has been made in the treatment of NSCLC, particularly in targeted therapy and immunotherapy. Among them, neoadjuvant targeted therapy and neoadjuvant immunotherapy have shown remarkable clinical efficacy. Neoadjuvant targeted therapy has been introduced in previous sections (see "Brief Summary of Neoadjuvant Targeted Therapy Research Progress in NSCLC").
Neoadjuvant immunotherapy for lung cancer mainly refers to the use of immune checkpoint inhibitors (ICIs) to eliminate the immune suppression of tumor cells on T cells by blocking immune checkpoint molecules such as PD-1/PD-L1/CTLA-4, and thereby reactivating T cells to clear tumor cells [2]. Currently, clinical research on neoadjuvant immunotherapy for NSCLC has become a hot topic, and clinical schemes include PD-1 inhibitor monotherapy, PD-L1 inhibitor monotherapy, immunotherapy combined with chemotherapy, and ICI dual therapy. The following will introduce each treatment scheme.
PD-1 inhibitor monotherapy
01 Nivolumab
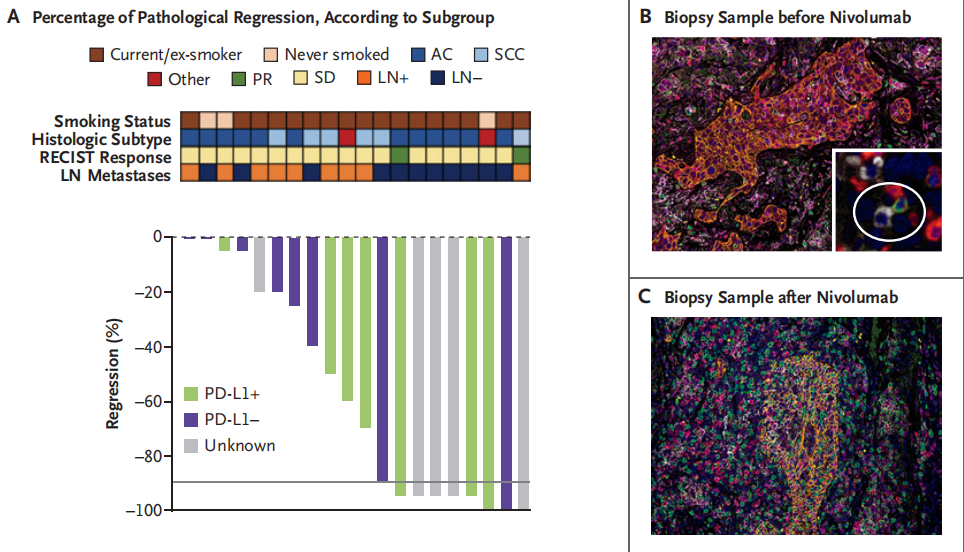
A single-arm, phase II study CheckMate-159 (NCT02259621) enrolled 21 resectable stage I-III A NSCLC patients. This study was one of the earliest small sample randomized controlled trials conducted in resectable stage I-III A NSCLC patients to evaluate the safety and efficacy of Nivolumab neoadjuvant therapy. Results showed that 20 out of 21 patients achieved curative resection, with 9 (45%) showing major pathological response (MPR) and 8 (40%) showing downstaging. 16 patients (80%) had no recurrence within 12 months after surgery. Nivolumab neoadjuvant therapy had fewer toxic side effects, achieved significant pathological response, and did not delay surgery.
02 Pembrolizumab
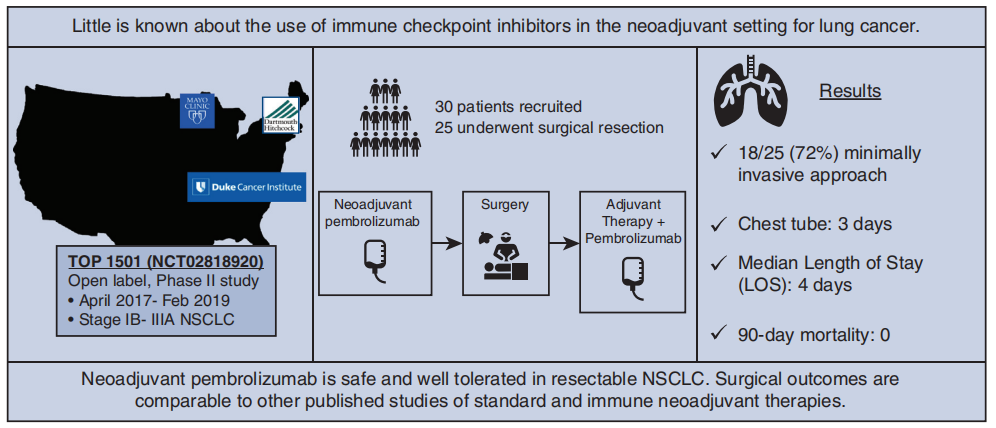
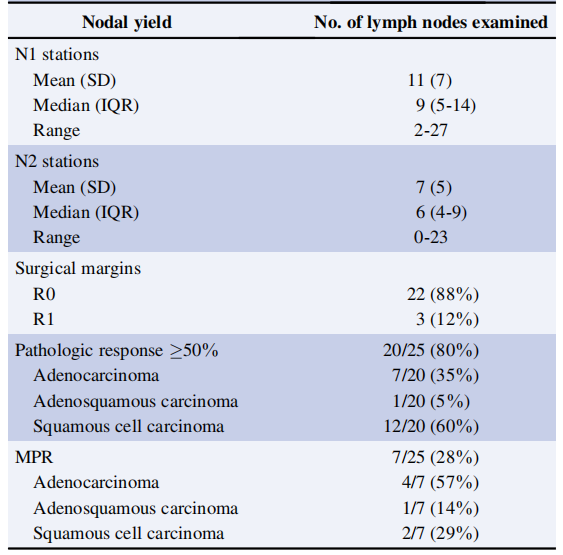
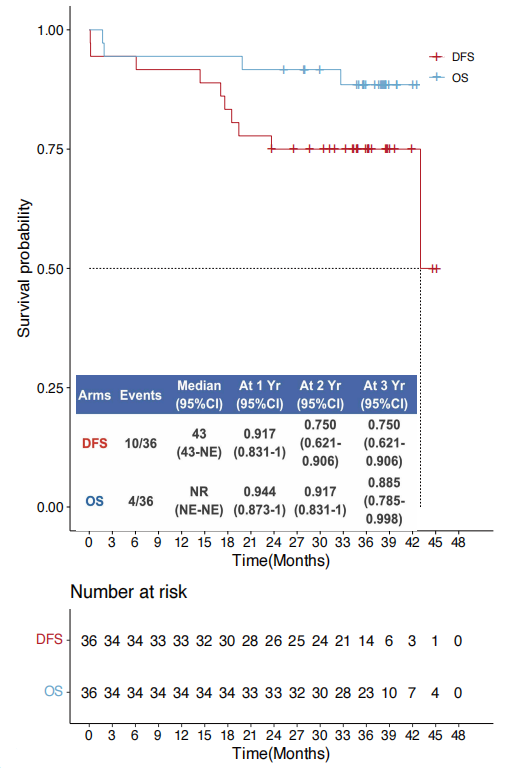
A prospective, phase II study TOP1501 (NCT02818920) enrolled 35 untreated stage IB-III A NSCLC patients to evaluate the safety and efficacy of Pembrolizumab neoadjuvant therapy in stage IB-III A NSCLC. Results showed that 30 patients were eventually enrolled (5 were excluded due to disease progression and ineligibility for surgery), with 11 (44%) patients showing downstaging, 22 (88%) achieving R0 resection, over 80% of tumor pathological response exceeding 50%, 28% of patients showing major pathological response (MPR), and 12% of patients achieving pathological complete response (pCR). Pembrolizumab neoadjuvant therapy was safe and well-tolerated, and was not associated with significant toxicity, preoperative delay, or additional surgical morbidity and mortality.
03 Sintilimab
A phase I study (registration number: ChiCTR-OIC-17013726) enrolled 40 resectable NSCLC patients with stage IA-III B to evaluate the safety and efficacy of Sintilimab in neoadjuvant therapy. This was the first study to report on the long-term survival probability of NSCLC patients receiving PD-1 inhibitors as neoadjuvant therapy. Results showed that 37 patients (92.5%) underwent surgery, with 36 achieving R0 resection. Ultimately, 15 patients (40.5%) achieved MPR, and 6 patients (16.2%) achieved pCR. The mean follow-up was 37.8 months, and the 3-year overall survival rate and disease-free survival rate for R0 resection patients were 88.5% and 75.0%, respectively. Sintilimab as a neoadjuvant therapy for NSCLC was well-tolerated, and MPR could reach 40.5%.
PD-L1 Inhibitor monotherapy
Atezolizumab
An ongoing phase II, open-label, single-arm LCMC3 study (NCT02927301) enrolled a total of 181 stage IB-III B NSCLC patients without EGFR or ALK mutations to evaluate the efficacy of Atezolizumab in neoadjuvant therapy. Results showed that among the 143 patients included in the primary endpoint analysis, the MPR was 20%, and the 3-year DFS and OS rates were 72% and 80%, respectively. The study demonstrated that Atezolizumab neoadjuvant therapy was safe and feasible, and achieved the primary endpoint of MPR≥15%.
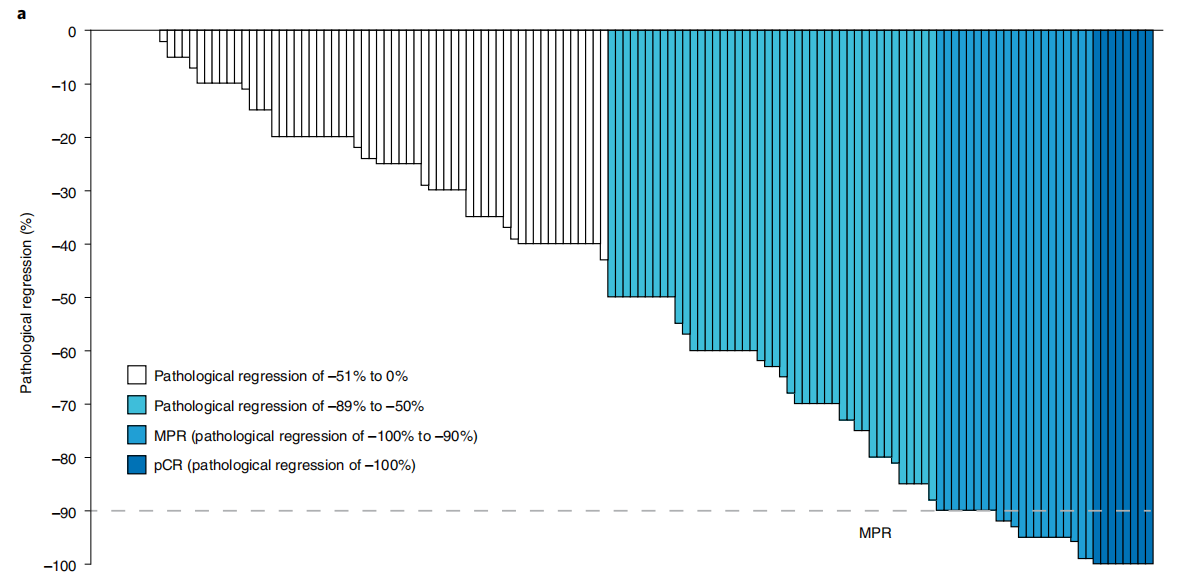
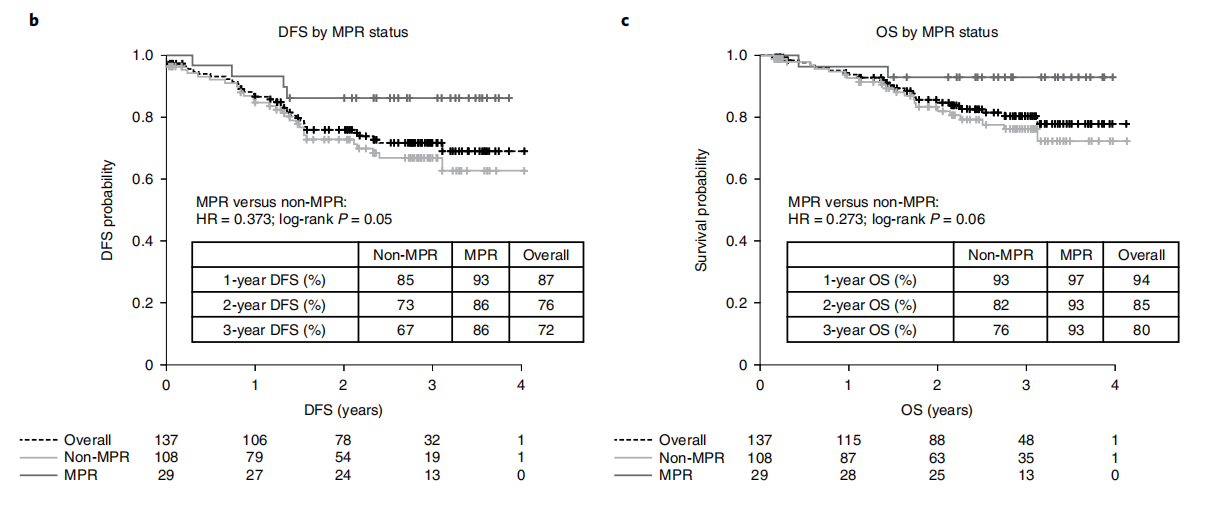
Immunochemotherapy
01 PD-1 inhibitor + chemotherapy
1)Nivolumab
A multicenter, open-label, phase III CheckMate 816 study (NCT02998528) enrolled 358 newly diagnosed resectable stage IB-III A NSCLC patients to evaluate the efficacy and safety of Nivolumab plus chemotherapy neoadjuvant therapy compared to chemotherapy alone. Results showed that 83.2% of patients in the Nivolumab plus chemotherapy neoadjuvant therapy group (experimental group) completed surgery, with 83.2% achieving curative resection, while only 75.4% of patients in the chemotherapy alone neoadjuvant therapy group (control group) completed surgery, with a curative resection rate of approximately 77.8%. The median EFS was 31.6 months in the experimental group and 20.8 months in the control group. The MPR and pCR rates were 36.9% and 24.0%, respectively, in the experimental group, compared to 8.9% and 2.2%, respectively, in the control group. Neoadjuvant therapy with Nivolumab plus chemotherapy significantly prolonged EFS and increased the proportion of patients achieving pathological complete response compared to chemotherapy alone in resectable NSCLC patients, without increasing the incidence of adverse events or hindering the feasibility of surgery. Based on the results of this study, on March 4, 2022, the FDA approved the use of Nivolumab plus platinum-based doublet chemotherapy as neoadjuvant therapy for resectable (tumor ≥ 4 cm or lymph node positive) NSCLC in adult patients (Q3W×3 cycles). On January 17, 2023, NMPA approved Nivolumab for neoadjuvant therapy in NSCLC. Nivolumab became the first ICI approved for neoadjuvant therapy in NSCLC.
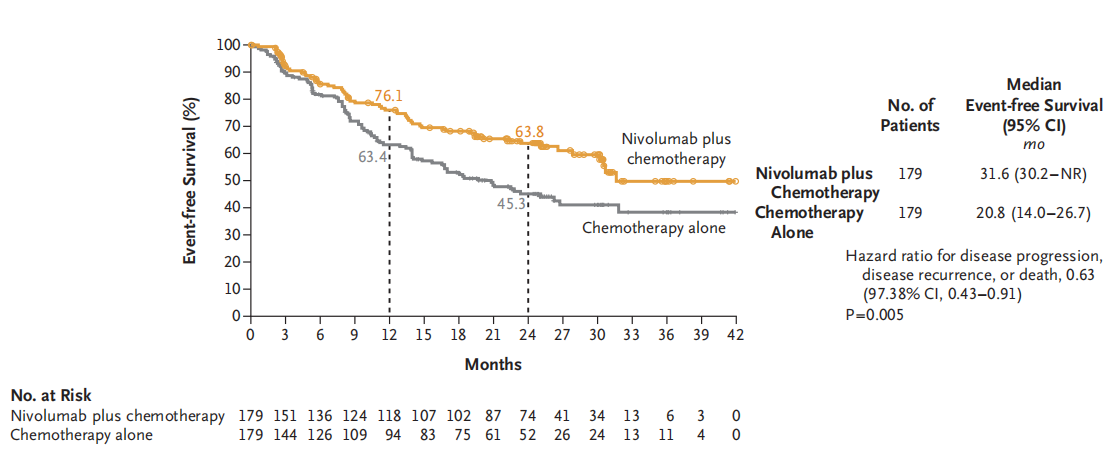
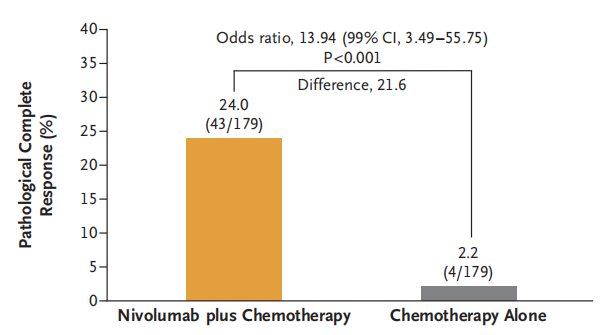
2)Toripalimab
An ongoing single-arm, phase II NeoTAP01 study (NCT04304248) enrolled 33 stage IIIA or T3-4N2 stage IIIB NSCLC patients without EGFR mutations or ALK fusion to evaluate the efficacy and safety of teriparatide monoclonal antibody combined with chemotherapy as neoadjuvant therapy for Asian patients with stage III NSCLC. Results showed that 30 patients underwent surgery, with 29 (96.7%) achieving R0 resection; 20 patients (66.7%) achieved MPR, of which 15 (50.0%) achieved pCR, and 24 patients (80.0%) achieved downstaging. The latest data from the study were presented at the 2022 ESMO Congress [10] (as of April 15, 2022). The median follow-up time for the ITT population (33 patients) was 16.4 months, and the median EFS had not yet been reached. The 12-month and 24-month EFS rates were 87.8% and 67.9%, respectively. MPR patients had significantly prolonged EFS compared to non-MPR patients, with 18-month EFS rates of 95.0% and 49.5%, respectively.
02 PD-L1 inhibitor + chemotherapy
1)Atezolizumab
An open-label, multicenter, single-arm phase II clinical trial (NCT02716038) enrolled 30 evaluable stage IB to IIIA NSCLC patients to evaluate whether the addition of Atezolizumab to chemotherapy improves patient outcomes. Results showed that 29 patients underwent surgery, with 26 (87%) achieving R0 resection; 17 (87%) achieved MPR and 10 (33%) achieved pCR. Neoadjuvant therapy with Atezolizumab plus chemotherapy was effective in treating resectable NSCLC, with a high proportion of patients achieving major pathological response, and manageable treatment-related toxicity that did not affect surgical resection.
2)Durvalumab
An ongoing randomized, double-blind, multicenter, global phase III AEGEAN study (NCT03800134) has enrolled 802 resectable stage IIa-IIIb NSCLC patients without EGFR or ALK gene mutations and regardless of PD-L1 expression to evaluate the efficacy of Durvalumab plus chemotherapy as neoadjuvant treatment. Although the perioperative immunotherapy AEGEAN study has already reported positive results, the specific study results are expected to be released in the first half of 2023.
03 CTLA-4 inhibitor + chemotherapy
Ipilimumab
A single-arm phase II TOP 1201 study (NCT01820754) enrolled 24 stage IB-IIIa NSCLC patients, with the first phase consisting of neoadjuvant chemotherapy alone, and the second and third phases consisting of chemotherapy combined with Ipilimumab. The study aimed to investigate the immunological effects of neoadjuvant chemotherapy combined with Ipilimumab in early-stage NSCLC patients. Results showed that 58% of patients had a partial response, 8% had disease progression, and the remaining patients had stable disease. The median overall survival was 29.2 months, and the 24-month overall survival rate was 73.0%. Ipilimumab demonstrated immune-activating properties in early-stage NSCLC.
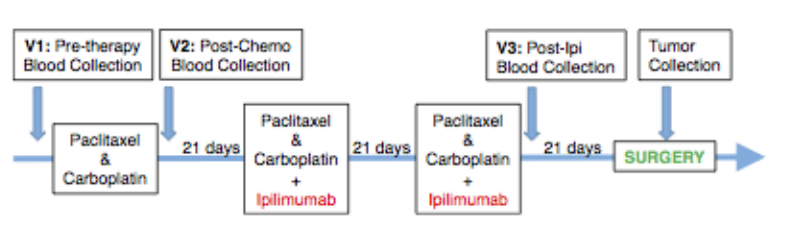
ICls combination therapy
Ipilimumab+Nivolumab
An open-label randomized phase II NEOSTAR study (NCT03158129) enrolled 44 stage I-IIA NSCLC patients to evaluate the efficacy of the PD-1 inhibitor Nivolumab combined with the CTLA-4 inhibitor Ipilimumab compared to Nivolumab monotherapy. Results showed that 39 patients completed surgery with R0 resection achieved in all cases. The MPR and pCR rates for Nivolumab monotherapy group were 22% and 9%, respectively, while those for Nivolumab plus Ipilimumab group were 38% and 29%, respectively. As of the publication of the data, median overall survival (OS) and relapse-free survival (RFS) had not been reached. Neoadjuvant treatment with Nivolumab plus Ipilimumab produced higher MPR and pCR rates, enhanced tumor immune infiltration, and induced immune memory.



Summary
In addition to ICIs mentioned above, there is a group of ICI new drugs in clinical trials, such as the CTLA-4 inhibitor Tremelimumab, LAG-3 inhibitor LAG525, TIM-3 inhibitor MBG-453, TIGIT inhibitor MTIG7192A, etc. It is expected that more prominent clinical results will emerge, which can improve the quality of life for patients. The emergence of neoadjuvant immunotherapy has provided a safe and feasible treatment option for NSCLC, benefiting patients at all stages of treatment. Treatment options such as dual immunotherapy and immunotherapy combined with chemotherapy have further provided patients with more choices and opportunities. Targeted and immune therapies have expanded new ideas and provided new means and hopes for traditional treatment options. Although the efficacy of both single-agent and combined traditional treatment is still in the exploratory stage and faces many challenges, their potential cannot be underestimated. With the continuous deepening of clinical research, it is believed that more locally advanced patients with potential for resection can improve their tumor resection rate and prolong survival through effective neoadjuvant treatment.
References
[1].CA Cancer J Clin,2020,70(1):7-30.
[2].Clin Cancer Res ,2019 ,25(19):5743-5751.
[3].N Engl J Med, 2018, 378(21): 1976-1986.
[4].J Thorac Cardiovasc Surg, 2022, 163(2): 427-436.
[5].J T horac Oncol, 2020, 15(5): 816 -826.
[6].Journal of Thoracic Oncology: Official Publication of the International Association for the Study of Lung Cancer, 2022: S1556-0864 (22) 00216.
[7].Nat Med. 2022 Oct;28(10):2155-2161.
[8].N Engl J Med 2022; 386:1973-1985.
[9].OncoImmunology, 2021, 10(1): 1996000.
[10]. ESMO 2022.955P.
[11]. Lancet Oncol, 2020, 21(6): 786-795.
[12] . Clin Lung Cancer, 2021, 23(3): e247-e251.
[13] .Clin Cancer Res, 2017, 23(2 4): 7474 -7482 .
[14]. Nat Med, 2021, 27(3): 504-514.
——This article is intended to provide scientific information to medical professionals only