Current location: Home > NEWS > Industry news
NEWS
PRODUCTS
A brief review for the research progress of neoadjuvant targeted therapy for NSCLC
News source: Release time:[2023-03-22]
Background Introduction
Lung cancer is the most common cancer in the field of tumors, and it is also the malignant tumor with the highest morbidity and mortality in the world. The most common type of lung cancer is non-small cell lung cancer (NSCLC), accounting for 80% to 85% of all lung cancers. Due to its low degree of malignancy, the growth and division of cancer cells is relatively slow, and there is little spread and metastasis in the early stage. It is not until the tumor enlarges to compress the surrounding tissues that clinical symptoms such as cough, bloody sputum, and chest pain gradually appear. Therefore, more than 30% of patients were diagnosed as locally advanced (stage III) at the time of initial treatment [1]. Surgical treatment is still the first choice for NSCLC, especially for stage III NSCLC, which is the most heterogeneous category in clinical practice. However, the effect of simple surgery is still not optimistic, especially in N2 stage, where the local recurrence rate and distant metastasis rate can reach as high as 20% and over 50% respectively [2]. Therefore, perioperative adjuvant therapy is particularly important. Targeted therapy has a significant effect on NSCLC cells, especially the third-generation EGFR-TKIs play an important role in the first-line/later-line and postoperative adjuvant therapy. But can targeted therapy be used as a preoperative neoadjuvant therapy? What is the current progress of neoadjuvant targeted therapy research? This article summarizes a series of studies for a brief discussion.
Meta-Analysis
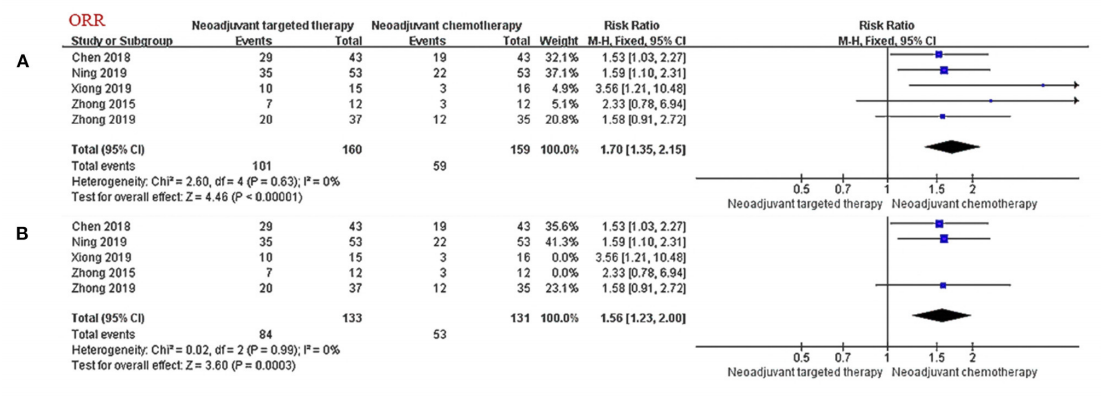
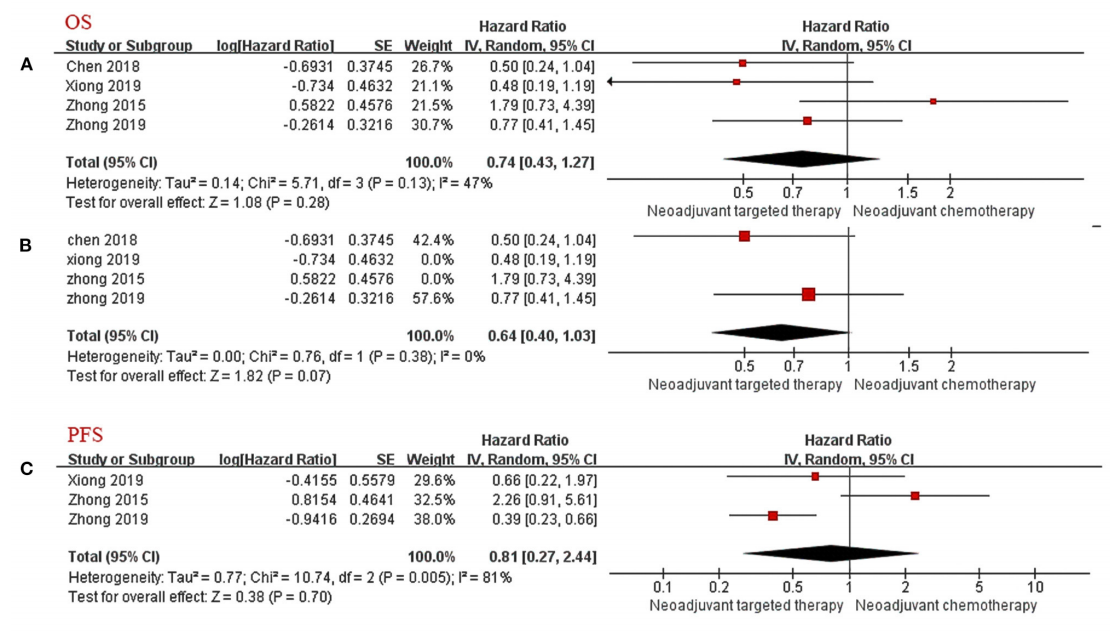
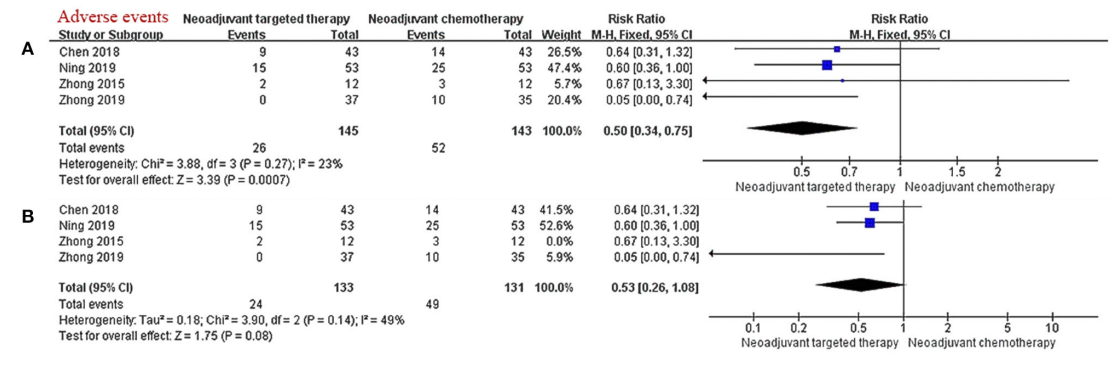
A meta-analysis [3] retrieved clinical trials on neoadjuvant targeted therapy published before October 2020, exploring the efficacy/safety and prognosis of neoadjuvant targeted therapy based on neoadjuvant chemotherapy. The results showed that: in the stage of neoadjuvant therapy, targeted therapy has a clear curative effect on patients with stage IIIA NSCLC with EGFR mutation (significantly improved ORR), and it is superior to chemotherapy in terms of safety and tumor response rate, but it is inferior in terms of OS and PFS, with significant difference has not yet been reached.
Erlotinib
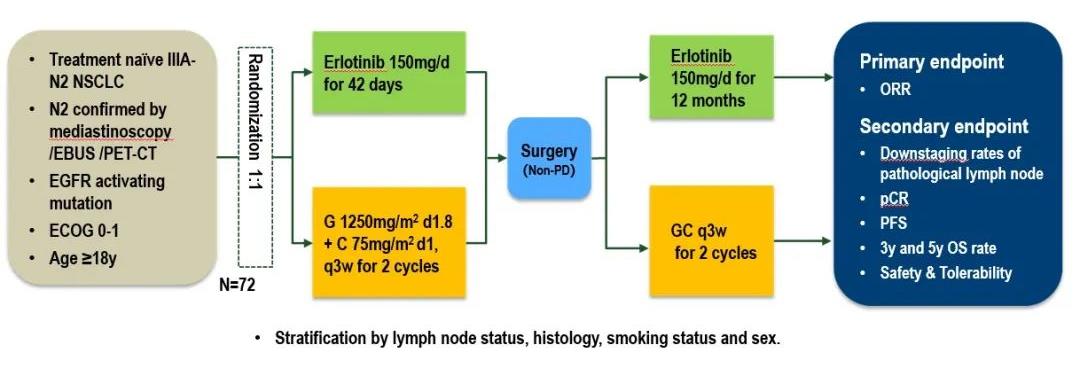
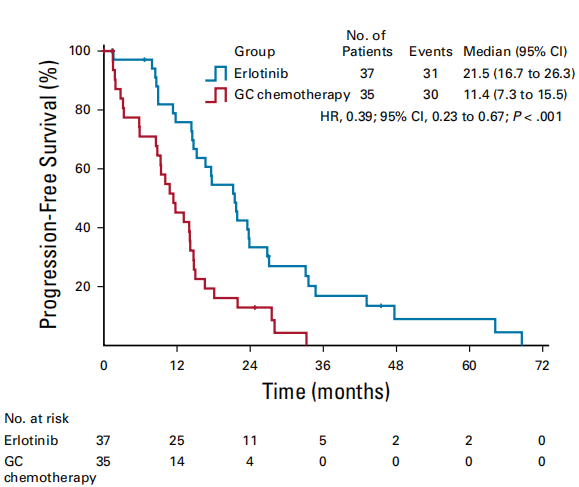
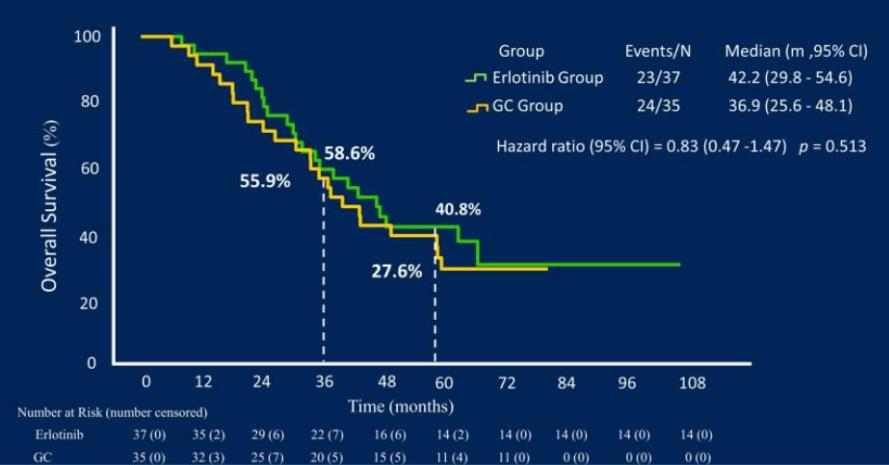
An open-label, prospective, randomized controlled phase II CTONG1103 study (NCT01407822) [4] from 17 centers in China compared the efficacy and safety of adjuvant therapy for Erlotinib with Gemcitabine + Cisplatin (GC regimen) as a new treatment for patients with EGFR sensitive mutation IIIA-N2 NSCLC.. The results showed that the primary endpoint ORR of the Erlotinib group and the GC group were 54.1% and 34.3% respectively (P=0.092); 9.7% of the patients in the Erlotinib group achieved MPR, while none in the GC group. At the same time, the median PFS of the Erlotinib group was significantly longer than that of the GC group, reducing the risk of progression or death by 61% (21.5 months vs 11.4 months, p<0.001). At the 2021 ASCO conference, the final OS data [5] was announced: the median follow-up time was 62.5 months, the median OS of the Erlotinib group and the GC group were 42.2 months and 36.9 months, respectively, and the 5-year OS rates were 40.8% and 27.6% (P=0.252). Erlotinib is feasible as neoadjuvant/adjuvant therapy for resected IIIA-N2 NSCLC with good OS, but the PFS survival advantage of Erlotinib did not translate into OS benefit.
Osimertinib
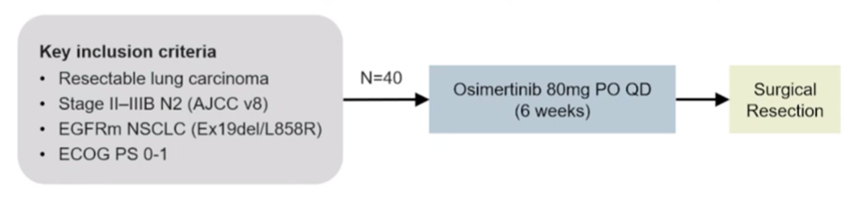
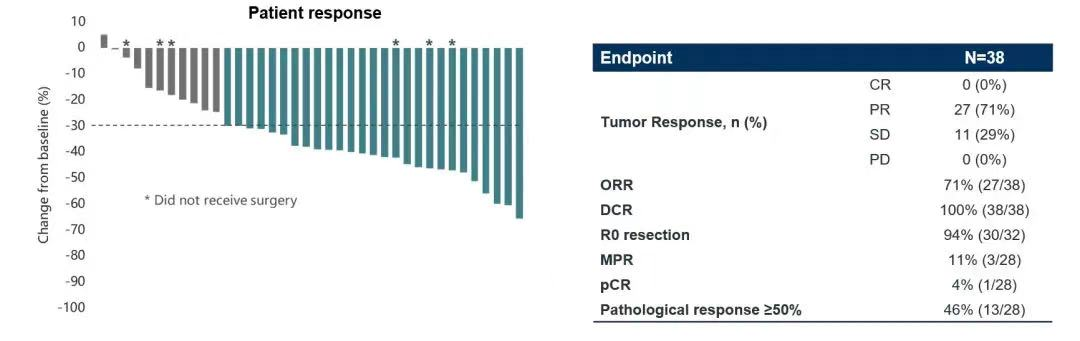
A prospective, multi-center, single-arm, phase II NEOS study (ChiCTR1800016948) led by Chinese scholars is the largest three-generation EGFR-TKI neoadjuvant therapy study so far, aiming to evaluate Osimertinib as a neoadjuvant therapy in Efficacy and safety for resectable EGFR-mutated (19del/L858R) lung adenocarcinoma. The 2022 European Lung Cancer Conference (ELCC) announced the latest NEOS study results [6], the study showed that the objective response rate (ORR) of Osimertinib neoadjuvant therapy reached 71.1%, the R0 resection rate reached 94%, and 46% of patients had pathological response ≥ 50%, the major pathological response rate (MPR) reached 11%, and 1 patient (4%) achieved a pathological complete response (pCR), and the safety was consistent with previous Osimertinib studies. Osimertinib, a third-generation EGFR-TKI as a neoadjuvant therapy, has achieved better curative effect, achieved tumor shrinkage in a short period of time, and improved the rate of complete surgical resection, which is expected to improve the long-term survival of patients.
Gefitinib
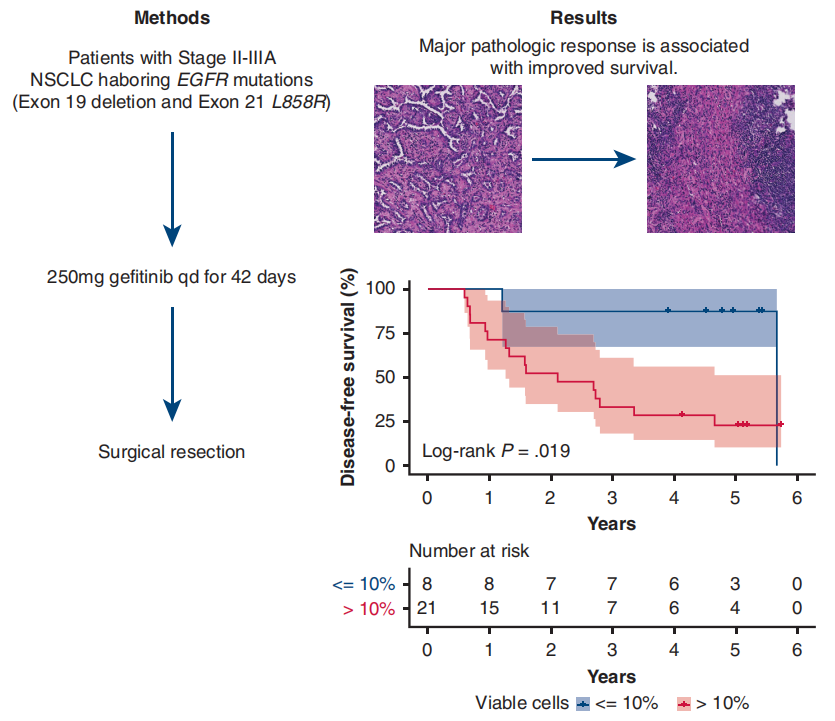
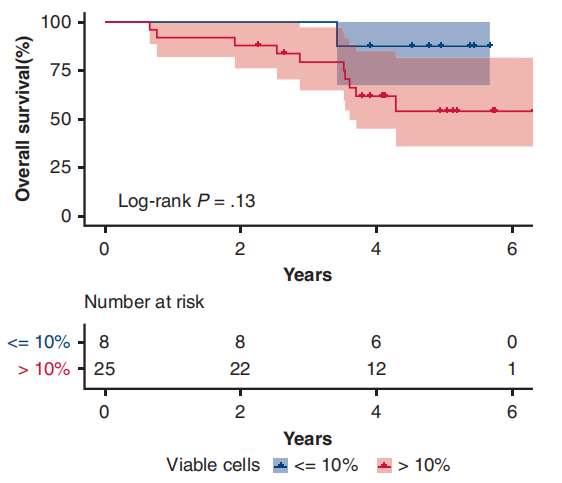
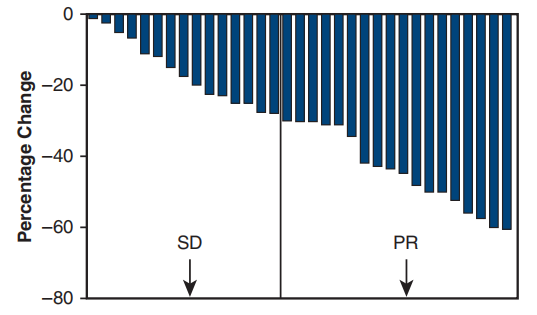
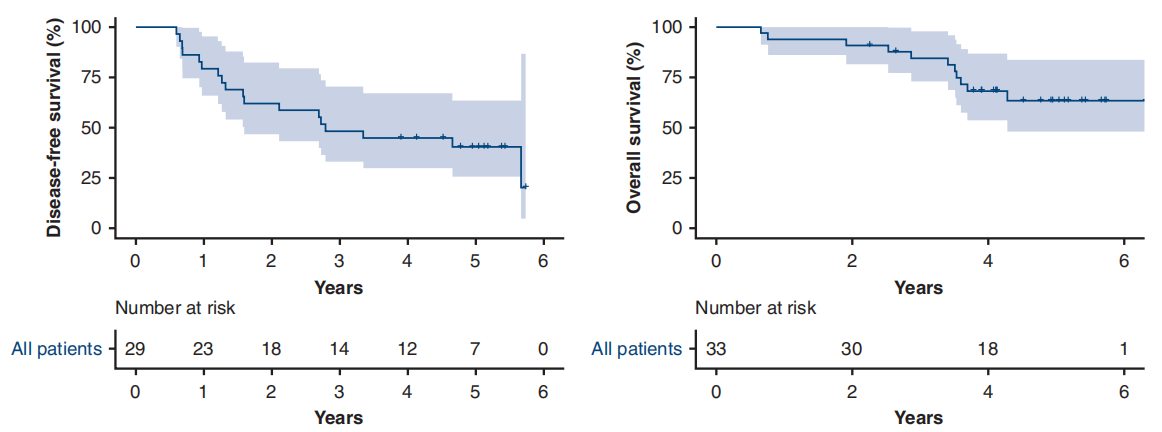
A single-arm phase II study (NCT01833572) [7] conducted at Shanghai Cancer Center aimed to investigate the efficacy and safety of preoperative Gefitinib in patients with stage II-IIIA operable NSCLC. The results showed that the primary endpoint ORR was 54.5%, the major pathological response (MPR) rate was 24.2%, and the median disease-free survival (DFS) was as high as 33.5 months, but the median overall survival (OS) was not reached. Patients who achieved MPR could significantly prolong DFS (P=0.019), and there was a tendency to prolong OS. For patients with stage II-IIIA NSCLC, neoadjuvant therapy with Gefitinib is safe, and it may be a feasible treatment for tumor patients with EGFR mutations, the major pathological response is correlated with the prognosis of patients.
Afatinib
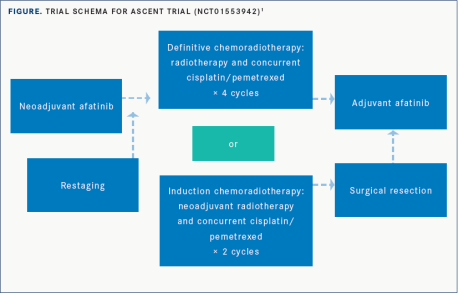
A prospective phase II clinical study Ascent (NCT01553942) [8] aims to explore the efficacy of Afatinib combined with standard neoadjuvant therapy. The enrolled patients first received 2 months of Afatinib treatment, and then graded according to the objective response rate (ORR), received 4 cycles of radiotherapy + Cisplatin + Pemetrexed or neoadjuvant radiotherapy + Cisplatin + Pemetrexed with 2 cycle then resection. The results showed that after 2 months of neoadjuvant Afatinib treatment, the ORR was 58%. The median PFS was 34.6 months, the median OS was 69.1 months, the 2-year OS rate was 88%. In stage III EGFR-mutant NSCLC, 2 months of neoadjuvant Afatinib was associated with comparable ORR in advanced disease and did not affect acceptance of standard chemoradiation ± surgery.
Crizotinib
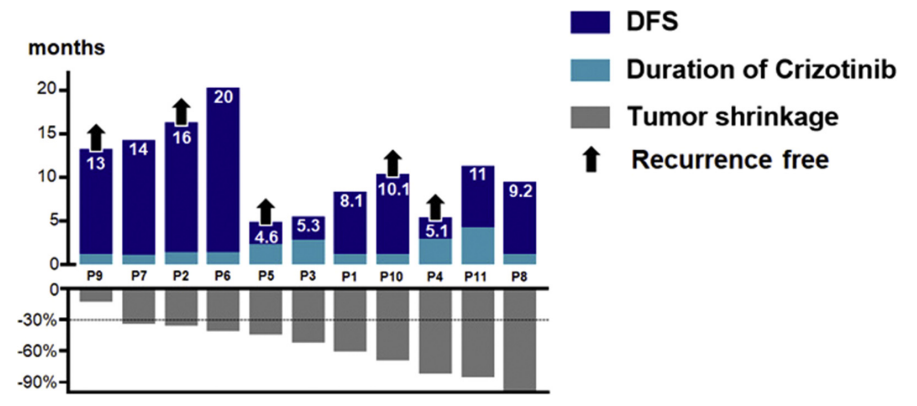
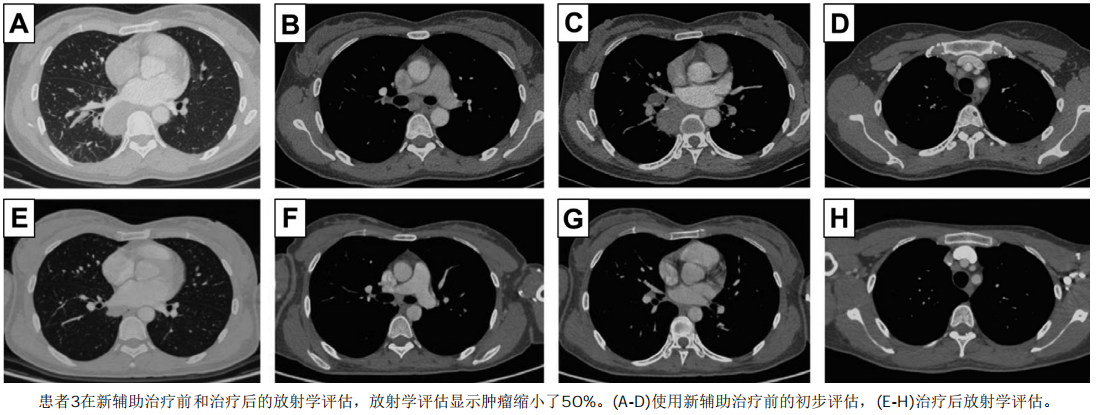
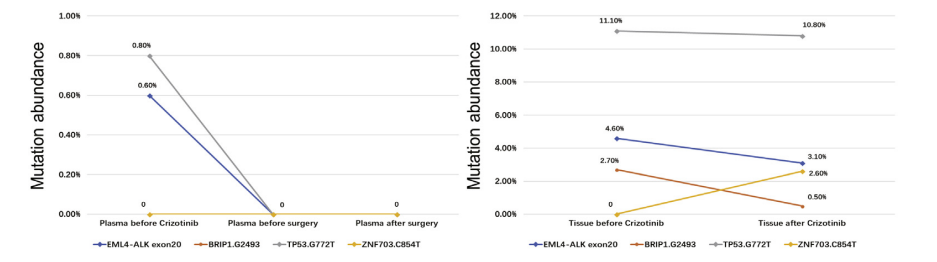
A retrospective study with a small sample size [9] evaluated the efficacy of Crizotinib in N2 stage NSCLC patients with ALK fusion before surgery. The results showed that: ORR was as high as 91.0%, 18.2% achieved complete remission (pCR), and 27.3% achieved N downstaging after Crizotinib neoadjuvant therapy. Simultaneous dynamic monitoring of plasma and tissue showed that the sensitive ALK signal decreased and partial response (approximately 50% of partial response) in patient 3 did not capture ALK-dependent drug resistance mutations. Complete resection of locally advanced disease may be feasible and well tolerated with neoadjuvant Crizotinib. Preoperative Crizotinib treatment can completely eliminate circulating molecular residual disease and will not affect the repeated use of first-line Crizotinib, but large-scale prospective trials are needed to prove its effectiveness in neoadjuvant therapy.
Ceritinib
A multi-center, single-arm, phase II SAKULA clinical trial [10] evaluated the efficacy and safety of Ceritinib as a preoperative neoadjuvant drug in the treatment of resectable ALK-positive locally advanced (LA) NSCLC. The results showed that the major pathological response rate (MPR) reached 57%, and 2 patients (29%) achieved complete remission. Ceritinib has certain clinical benefits in neoadjuvant therapy.
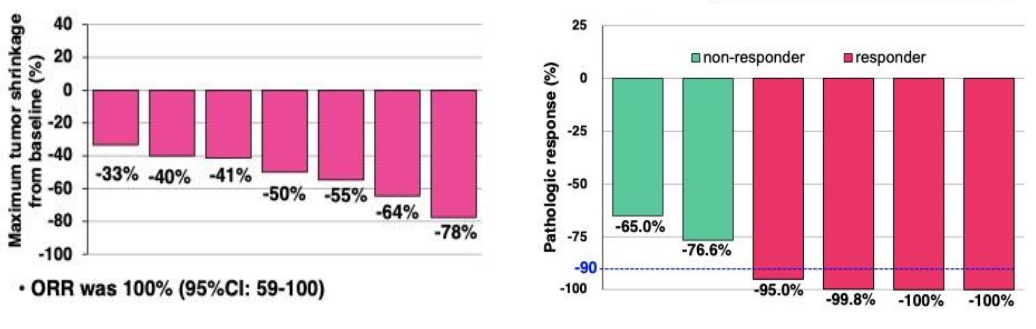
Alectinib
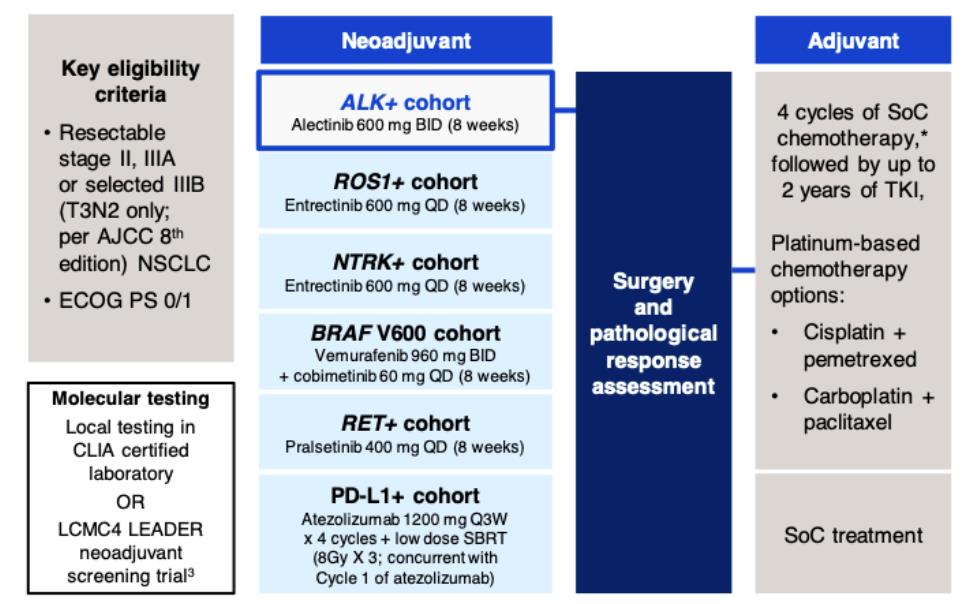
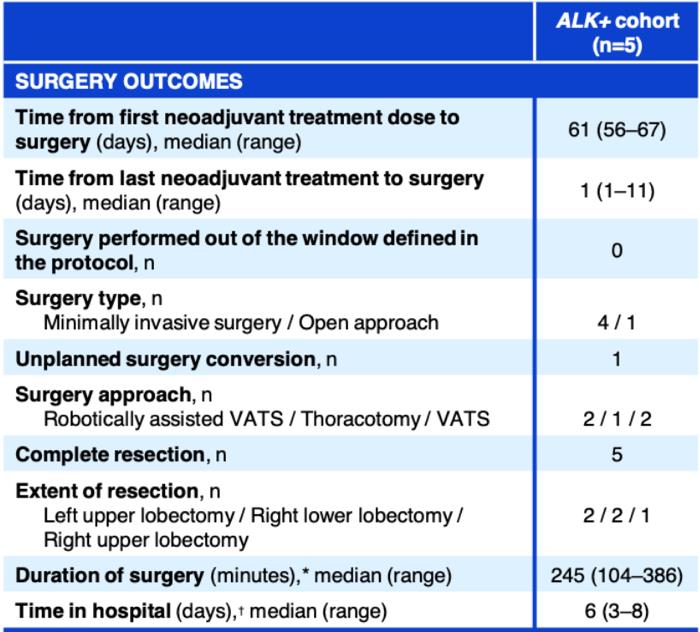
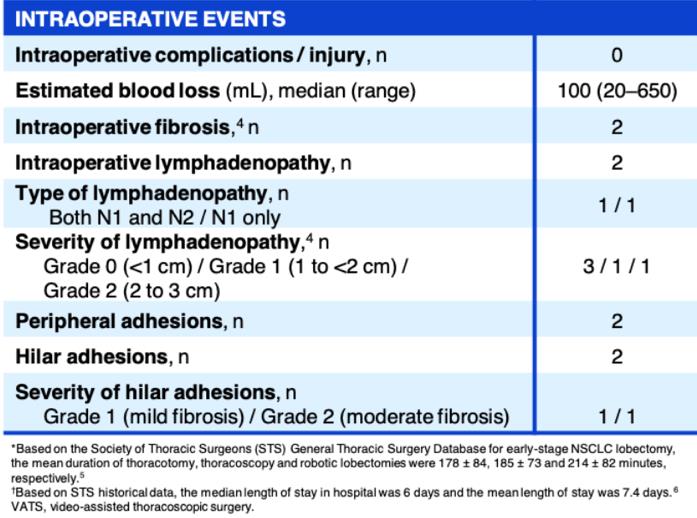
An ongoing phase II umbrella NAUTIKA1 study (NCT04302025) [11] aims to explore the efficacy and safety of Alectinib for neoadjuvant and adjuvant treatment of patients with stage II-III ALK+ resectable NSCLC. At the 2022 World Conference on Lung Cancer (WCLC 2022), the NAUTIKA1 study announced preliminary data on the ALK+ cohort in neoadjuvant therapy. As of May 9, 2022, a total of 8 ALK-positive patients were included in the study, and 5 of them completed neoadjuvant treatment with Alectinib. All patients completed the operation within the window period (day 57 ± 10 days), and the operation achieved R0 resection (R0 resection rate: 100%), without delay or serious surgical complications. In the primary analysis of the ALK+ cohort of the NAUTIKA1 study, Alectinib was well tolerated in patients with stage II-III ALK+ NSCLC and is a viable option for neoadjuvant therapy.
Summary
NSCLC is a heterogeneous disease at the molecular level. With the development of precision medicine, targeted therapy has gradually become the preferred option for targeted patients, and has become the direction of research and exploration for neoadjuvant therapy for patients who carried positive driver genes. Although neoadjuvant targeted therapy is not routinely recommended, a large number of clinical trials have shown good efficacy: for EGFR mutation populations, especially the high mutation rate of the Chinese population, whether in efficacy or safety, the neoadjuvant targeted therapy compared with neoadjuvant chemotherapy, has more advantages. But there are still many issues worthy of further research, such as the duration of TKI medication. Is there a benefit in overall survival? How to choose the drug regimen if there are coexisting mutant genes? Can TKI combined with chemotherapy or radiotherapy achieve greater efficacy and so on? Looking forward to more phase III clinical studies to obtain higher-level evidence. In addition, ALK is an important therapeutic target for advanced NSCLC and has become a research hotspot in neoadjuvant therapy. The entry of ALK-TKI into early-stage NSCLC has become an unstoppable trend. We look forward to the release of more experimental data or real-world data verification to provide more evidence for the clinical application of early-stage ALK patients.
Reference
[1].Frontiers in Oncology,2019,9:786.
[2].Chinese Journal of Oncology,2017,27(05):383 -388.
[3].Frontiers in Surgery, 2021, 8:342-.
[4].Journal of Clinical Oncology,2019,37(25):2235-2245.
[5].Journal of Clinical Oncology, 2021, 39(15_suppl) : 8502-8502.
[6].Updated results. ELCC 2022,81MO.
[7].J Thorac Cardiovasc Surg,2021,161(2):434-442.
[8].Journal of Thoracic Oncology, 2021, 16(3):S188.
[9].J Thorac Oncol 2019, 14(4):726-731.
[10].2021 WCLC Abstract WS06.03.
[11].Journal of Thoracic Oncology, 2022, 17(9S) : S233-S234.
——This article is only used to provide scientific information to medical professionals