Current location: Home > NEWS > Industry news
NEWS
PRODUCTS
Precision therapy target for Leukemia, BCR-ABL gene T315I mutation detection
News source: Release time:[2022-12-14]
Precision therapy target for Leukemia, BCR-ABL gene T315I mutation detection
A. Epidemiological Investigation
Interpretation of the 2020 Global Cancer Statistical Report, Volume 7, Issue 2, Electronic Journal of Comprehensive Cancer Therapy, 2021.
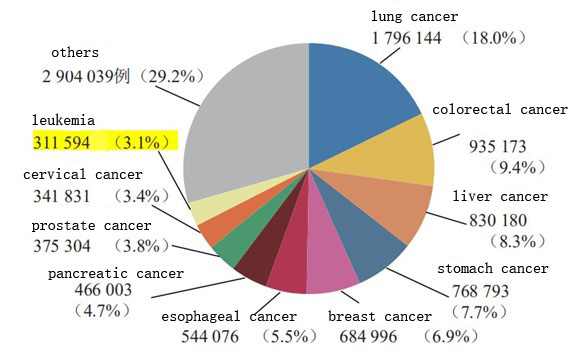
Estimated new deaths from cancer worldwide in 2020
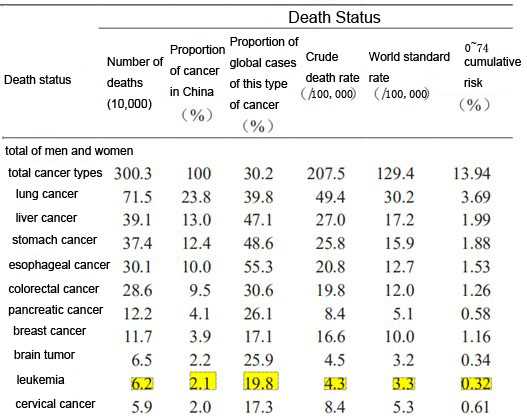
Statistics on common cancer deaths in China in 2020
B. leukemia
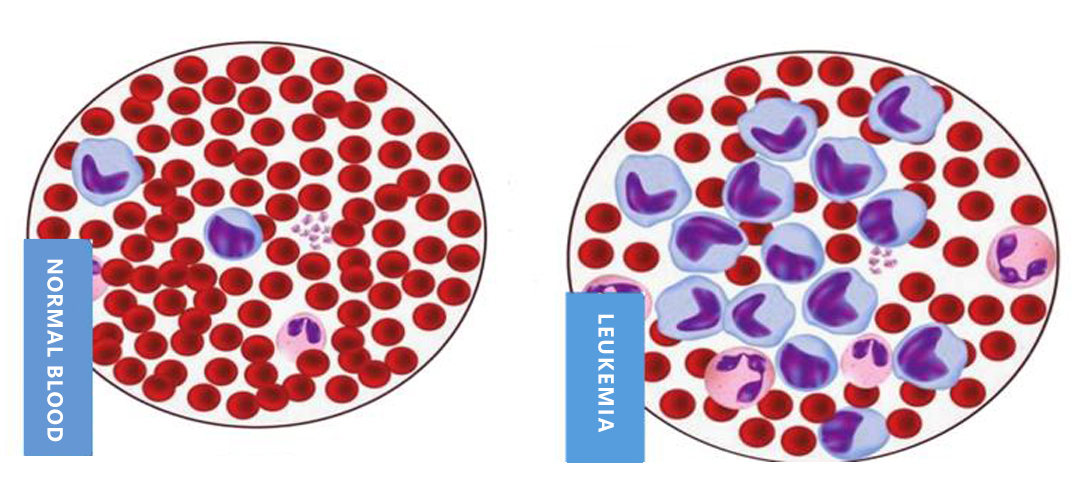
Normal blood and leukemia comparison chart
1)Leukemia is a malignant clonal disease of hematopoietic stem cells. It can be clinically manifested as fever, anemia, enlarged lymph nodes, fatigue, hyperhidrosis, etc. Due to the growth disorder of leukemia cells, white blood cells stagnate at different stages of cell development, and then in the bone marrow and other hematopoietic tissues, which affect normal hematopoiesis, and can be divided into acute leukemia and chronic leukemia clinically.
White blood cell proliferation and fission
2)There are many types of leukemia, the following are some common types:
①ALL,acute lymphoblastic leukemia:It is more common in children under 9 years old, and more than 20% of blast lymphocytes can be seen in bone marrow cell examination.
②Acute myeloid leukaemia (AML).
③CML,chronic myelocytic leukemia:More common in adults.
④Chronic lymphocytic leukemia (CLL)。
⑤Hairy cell leukemia.
⑥prolymphocytic leukemia.
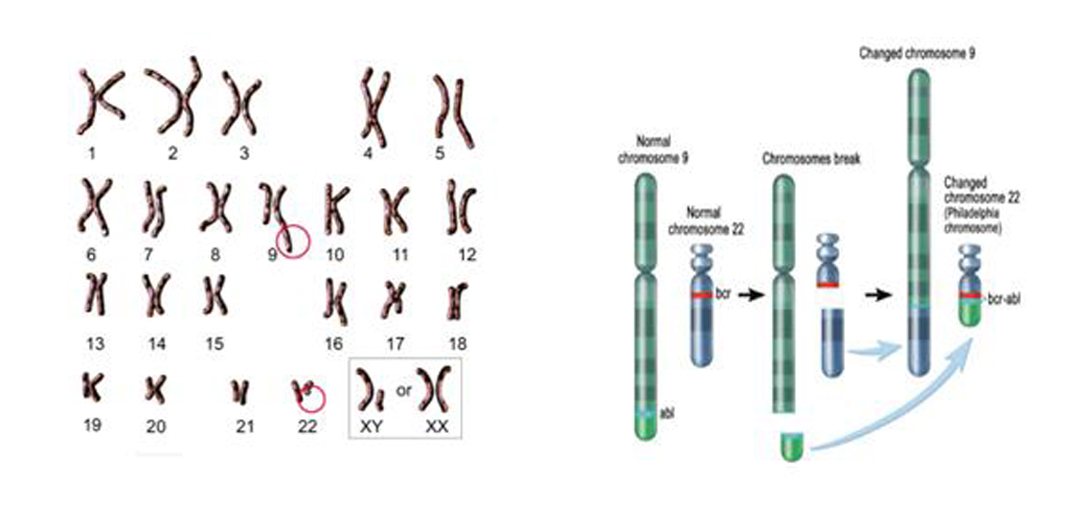
23 pairs of human chromosomes Fusion of BL and BCR genes
3)Chronic myelogenous leukemia(CML)
Also known as: chronic myeloid leukemia, is a malignant tumor formed by the clonal proliferation of bone marrow hematopoietic stem cells.
Features: The malignant transformation of myeloid cells in the bone marrow produces a large number of abnormal granulocytes-the increase of white blood cells.
The cause of the disease is related to chromosomal mutations in the body: translocation of chromosomes 9 and 22, resulting in fusion of ABL and BCR genes (one of the diagnostic criteria).
It is present in 95% of chronic myeloid leukemia (CML), 20%-30% of adult acute lymphoblastic leukemia (ALL) and 2%-5% of children with ALL.
4)Diagnosis and initial treatment of patients with chronic myeloid leukemia (CML) in the chronic phase:
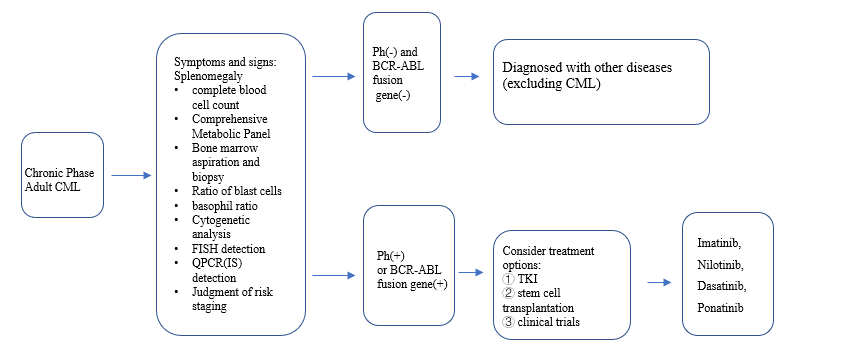
Note: IS (International Standardization) Ph (Philadelphia Chromosome) TKI (Tyrosine Kinase Inhibitor)
5)BCR-ABL and T315I mutation
a. Targeted therapy for BCR-ABL fusion detection
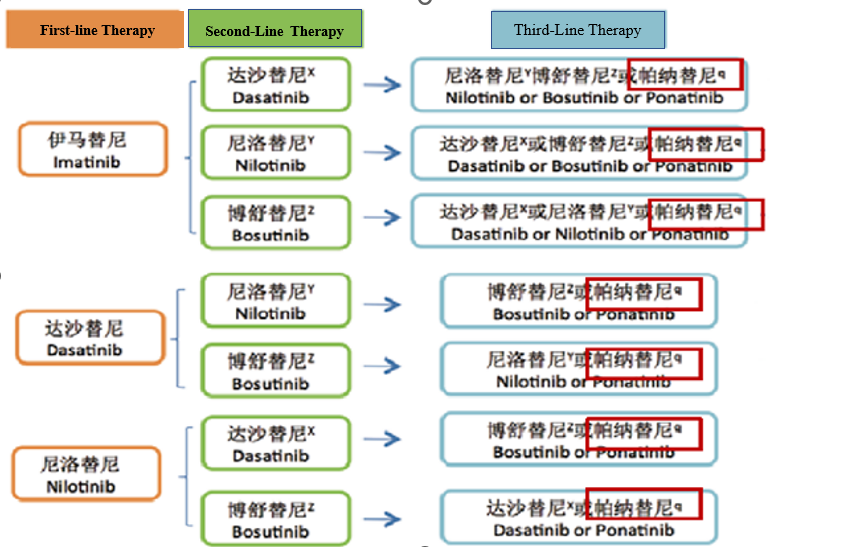
Targeted therapy for BCR-ABL fusion detection
b. T315I mutation in BCR-ABL gene:
For patients with T315I mutation, Imatinib, Dasatinib, and Nilotinib are not effective, and Ponatinib is recommended.
C. Related Clinical Research
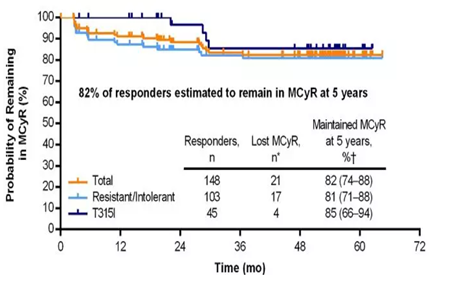
Figure 1: MCyR status in CP-CML patients
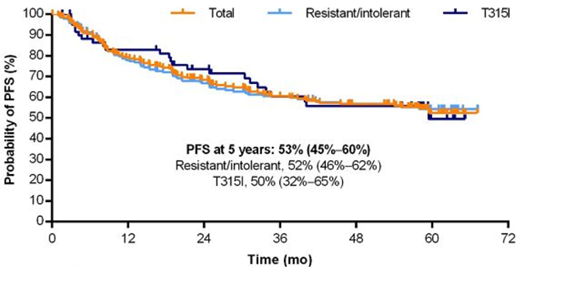
Figure 2: 5-year PFS in CP-CML patients
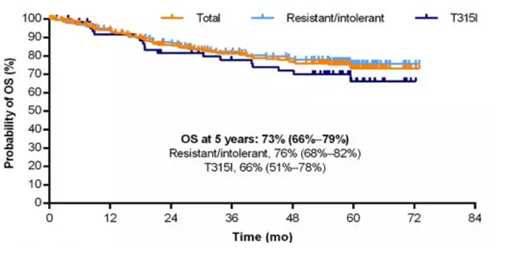
Figure 3: 5-year OS of CP-CML patients
Study data were available for 267 CP-CML patients:
①At 12 months, a total of 148 patients (55%) with CP-CML achieved major cytogenetic response (MCyR), and 82% of these patients could maintain MCyR to the 5-year follow-up endpoint (see Figure 1).
②In terms of survival, the 5-year PFS and OS of CP-CML patients were 53% and 73%, respectively (see Figures 2 and 3).
Conclusion: For CML patients with BCR/ABLT315I mutation, multiple TKIs including Imatinib, Dasatinib, Bosutinib and Nilotinib are usually resistant. Studies have shown that Ponatinib is currently the only effective TKI-targeted drug.
# SPACEGEN
Human BCR-ABL Gene T315I Mutation Detection Kit
Test content
Product | Detection Method | Pack Size | Compatible Instrument | Sample Type |
Human BCR-ABL Gene T315I Mutation Detection Kit | Multiplex fluorescent PCR | 20 tests/kit | Stratagene Mx3000P™, ABI7500 etc. | peripheral blood, bone marrow |
Applicable people and detection significance
Leukemia patients with Imatinib-resistant CML patients should be tested for BCR-ABL gene T315 mutation before changing TKI drugs, so as to provide reference for CML patients' medication.
Detection advantage
Stable and reliable
Closed tube detection, no need for post-product handling;
Easy Operation
One-step operation, only 90 minutes to finish the detection.
peripheral blood, bone marrow
Good repeatability
It can be carried out in ordinary PCR laboratories, and reproducible results can be obtained without special training;
Advanced Technology
Developed based on the proprietary PAP-ARMS® technology.
Detection Process
1.Nucleic Acid Extraction
2.Set up qPCR
3.Amplification
4.Data Analysis
Reference
[1] Interpretation of 2020 Global Cancer Statistical Report, Volume 7, Issue 2, Electronic Journal of Comprehensive Cancer Therapy, 2021.
[2] 2021 CSCO guidelines for blood disorders.
[3] Ponatinib efficacy and safety in Philadelphia chromosome-positive leukemia: final 5-year results of the phase 2 PACE trial.Blood 2018 Mar 22