Current location: Home > NEWS > Industry news
NEWS
PRODUCTS
Molecular mechanism and targeted therapy of low-grade serous ovarian carcinoma-part 2
News source: Release time:[2022-12-13]
part 2
Targeted therapy
Patients with LGSOC, especially those with advanced stage, are prone to relapse. For these patients, comprehensive somatic genetic testing is particularly important for the selection of treatment options [2]. Currently, some evidence supports the effectiveness of MEK inhibitors, BRAF inhibitors, and Bevacizumab in the treatment of LGSOC.
The phase II/III open-label, randomized GOG-0281 trial (NCT02101788) compared the MEK1/2 inhibitor Trametinib with standard care (Letrozole, liposomal Doxorubicin, weekly treatment of Paclitaxel, Tamoxifen or Topotecan) in the treatment of recurrent LGSOC, a total of 260 patients were included. Compared with the standard treatment group, Trametinib significantly prolonged the median progression-free survival (PFS) of patients with recurrent LGSOC (13.0 months vs 7.2 months, hazard ratio (HR)=0.48, P<0.0001). The objective response rate (ORR) was 26% vs 6% (odds ratio (OR) = 5.4, P < 0.0001), and an additional 59% of patients had stable disease for at least 8 weeks. The duration of tumor response was prolonged (13.6 months vs 5.9 months). The median overall survival (OS) was 37.6 months vs 29.2 months (HR=0.76, P=0.056), favoring Trametinib group. Based on the results of this clinical trial, NCCN guidelines recommend Trametinib for targeted therapy of recurrent LGSOC (category 2A) [2-3] [6].
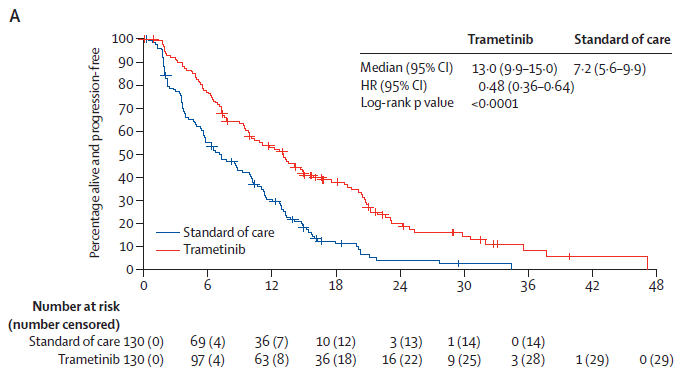
KM curve of primary endpoint PFS [6]
The phase III open-label MILO/ENGOT-ov11 study (NCT01849874) investigated the efficacy of Binimetinib versus physician-selected chemotherapy (PCC), including Paclitaxel, liposomal Doxorubicin and Topotecan, in 303 patients with relapsed LGSOC . The primary endpoint is PFS assessed by the blinded independent review committee (BICR). Binimetinib group vs. PCC group, the median PFS was 9.1 months vs. 10.6 months (HR=1.21, P=0.807), because the evaluation was difficult to achieve the main study outcome ,the trial was terminated early [7]. However, NCCN believes that the data such as PFS and ORR evaluated by researchers in the Binimetinib group are better than those in the PCC group, and KRAS mutations may predict the response to Binimetinib. In summary, the NCCN guidelines recommend Binimetinib for targeted therapy of recurrent LGSOC (category 2B) [2] [7].
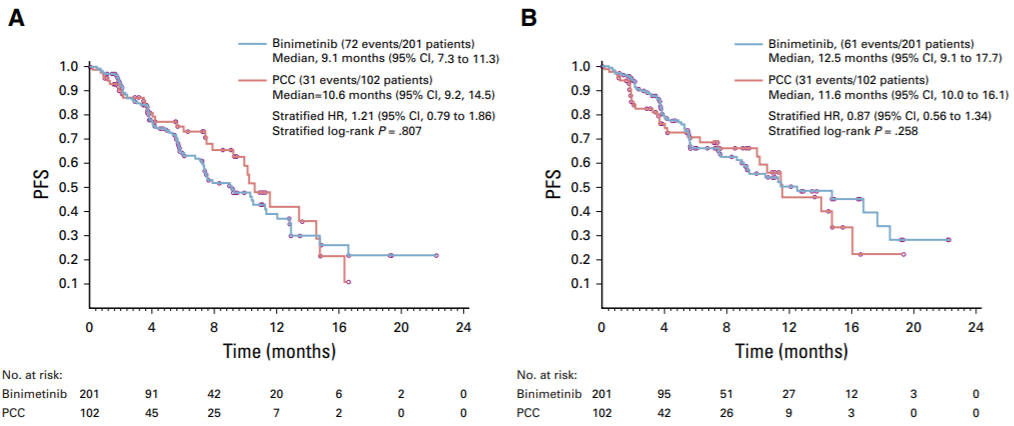
KM curves of PFS assessed by BICR (A) and investigators (B) [7]
Recently, patients with recurrent LGSOC have new options. In June this year, the FDA approved the BRAF inhibitor Dabrafenib combined with Trametinib for late-line treatment of patients with unresectable or metastatic solid tumors with BRAF V600E mutations. This approval is based on the results of multiple clinical trials, one of which is subprotocol H of the single-arm, open-label Phase II NCI-MATCH trial (EAY131-H, NCT02465060). The study included 6 patients with gynecological tumors, 5 of which were LGSOC, and the ORR reached 80%. The overall median PFS for the study was 11.4 months, and the median OS was 28.6 months. Accordingly, the NCCN guidelines recommend Dabrafenib combined with Trametinib for targeted therapy of recurrent LGSOC with BRAF V600E mutation (category 2A)[2][8][9].
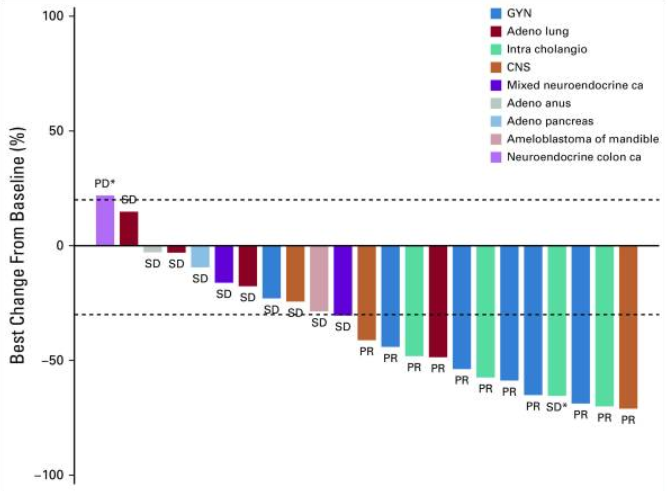
Waterfall plot of tumor volume change from baseline - Dabrafenib combined with Trametinib [9]
PARP inhibitors, which are widely used in HGSOC, are not included in the inclusion criteria of clinical trials in LGSOC patients [10]. It is also mentioned above that the molecular mechanism of LGSOC is different from that of HGSOC, and the HRD score is generally lower. Therefore, there is currently no clinical research data to support the efficacy of PARP inhibitors in LGSOC patients. However, it is also mentioned in the guidelines that first-line maintenance therapy with PARP inhibitors is mainly applicable to HGSOC and endometrioid carcinoma histological types, as well as other EOC histological types with BRCA1/2 mutations [11].
The FDA approved the trk inhibitors Larotrectinib and Entrectinib on November 26, 2018 and August 15, 2019, respectively, for the treatment of patients with unresectable or metastatic solid tumors carrying NTRK gene fusions [8]. Among them, the clinical study of Entrectinib included two patients with gynecological tumors, one with ovarian cancer and the other with endometrial cancer, both of which achieved partial response (PR) [12]. The NCCN guidelines recommend these two drugs for targeted therapy of NTRK fusion-positive recurrent ovarian cancer (category 2A) [2]. The NMPA approved the domestic marketing of two drugs on April 8 and July 26 this year, respectively, which increased the options for relapsed patients [13]. However, there are no data supporting the frequency of NTRK fusions and the efficacy of trk inhibitors in patients with LGSOC.
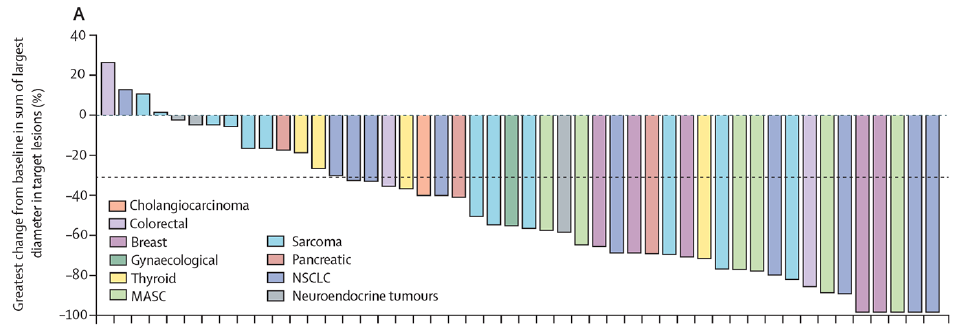
Waterfall plot of tumor volume change from baseline - Entrectinib [10]
The anti-angiogenic drug Bevacizumab has been approved for use in combination with chemotherapy followed by monotherapy as maintenance therapy for postoperative adjuvant treatment in patients with stage III to IV ovarian cancer, or in patients with platinum-sensitive recurrent ovarian cancer; and in combination with chemotherapy for platinum-resistant recurrent ovarian cancer patients [8]. There are also some retrospective studies on Bevacizumab for recurrent LGSOC, mainly in combination with chemotherapy, with an ORR of about 50%; studies on Bevacizumab alone are rare. In addition, the combination of multiple targeted drugs, such as EGFR inhibitors combined with MEK inhibitors, MEK inhibitors combined with PI3K/mTOR inhibitors, has also been studied [3]. Pembrolizumab and Dostarlimab,, the two immune checkpoint inhibitors (ICIs), have been approved as pan-solid tumor inhibitors, and ovarian cancer patients have been included in clinical trials. NCCN guidelines recommend these two drugs for recurrent ovarian cancer immunotherapy (category 2A) [2].
Summary
Although the incidence of LGSOC is much lower than that of HGSOC, its molecular pathways, biological behaviors, and clinical features are different from those of HGSOC, and the treatment of HGSOC cannot be simply copied. In addition to chemotherapy and endocrine therapy, MEK inhibitors, BRAF inhibitors, Bevacizumab, etc. are also commonly used in targeted therapy for patients with recurrent LGSOC; PARP inhibitors, trk inhibitors, ICIs, etc. may have potential application value.
Reference
[1]CSCO Ovarian Cancer Diagnosis and Treatment Guidelines 2020
[2] NCCN Clinical Diagnosis and Treatment Guidelines for Ovarian Cancer 2022 v3
[3] Expert consensus on low-grade serous ovarian cancer (2020 edition)
[4] 2020 WHO classification of female genital tumours
[5] J Pathol. 2021 Jan;253(1):41-54.
[6] Lancet. 2022 Feb 5;399(10324):541-553.
[7] J Clin Oncol. 2020 Nov 10;38(32):3753-3762.
[8] FDA Official website data
[9] J Clin Oncol. 2020 Nov 20;38(33):3895-3904.
[10] Clinical Trails Official website data
[11] Guidelines for Clinical Application of PARP Inhibitors in Ovarian Cancer
[12] Lancet Oncol. 2020 Feb;21(2):271-282.
[13] NMPA Official website data