Current location: Home > NEWS > Corporate news
NEWS
PRODUCTS
The sub medical laboratory of SapceGen passed the CAP BRCA-A 2022 proficiency assessment test with a perfect score
News source: Release time:[2022-12-05]
Recently, College of American Pathologists (CAP) announced the report of BRCA1/2 Sequencing (BRCA-A 2022) external quality assessment results. SpaceSeq MedLab passed the proficiency assessment test with a perfect score.
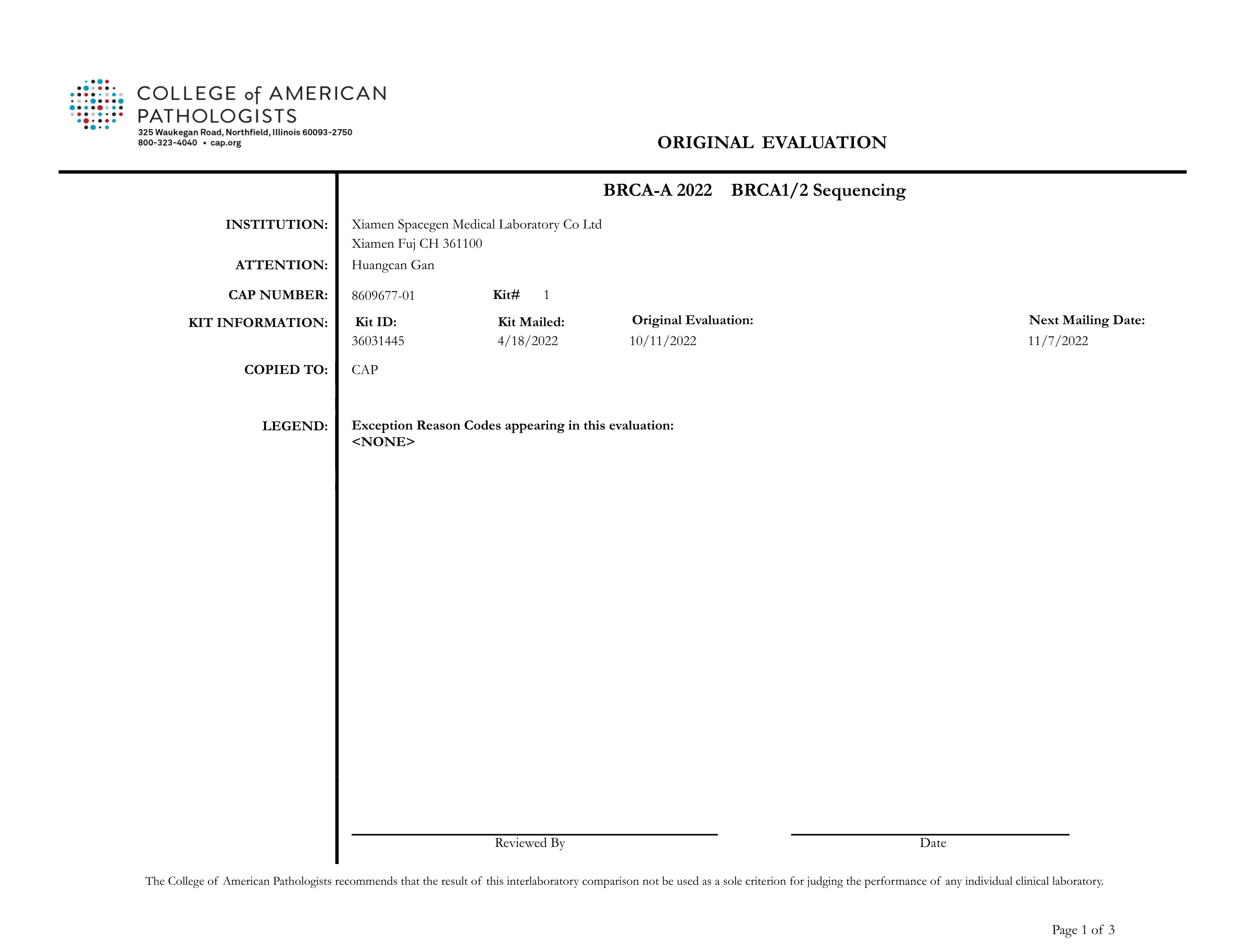
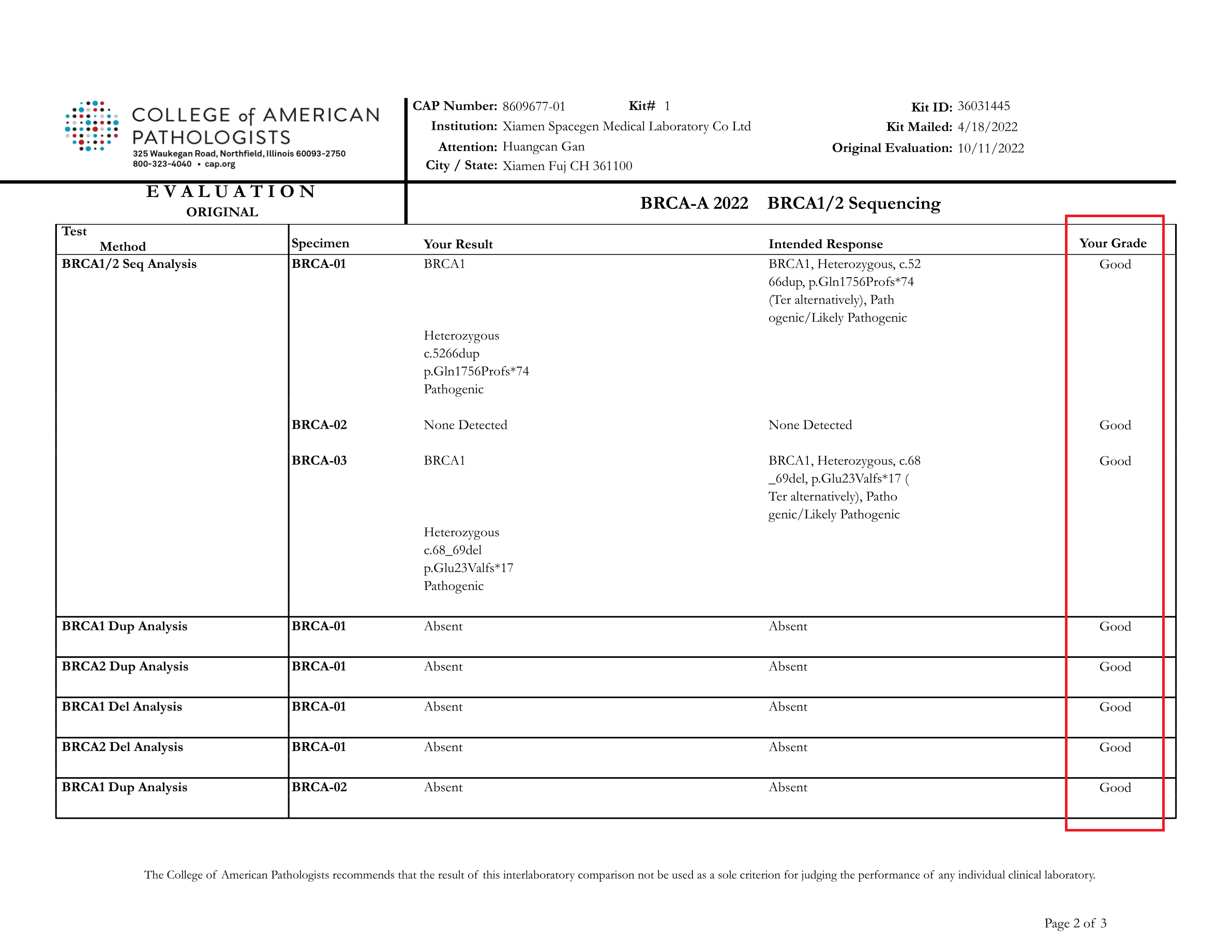
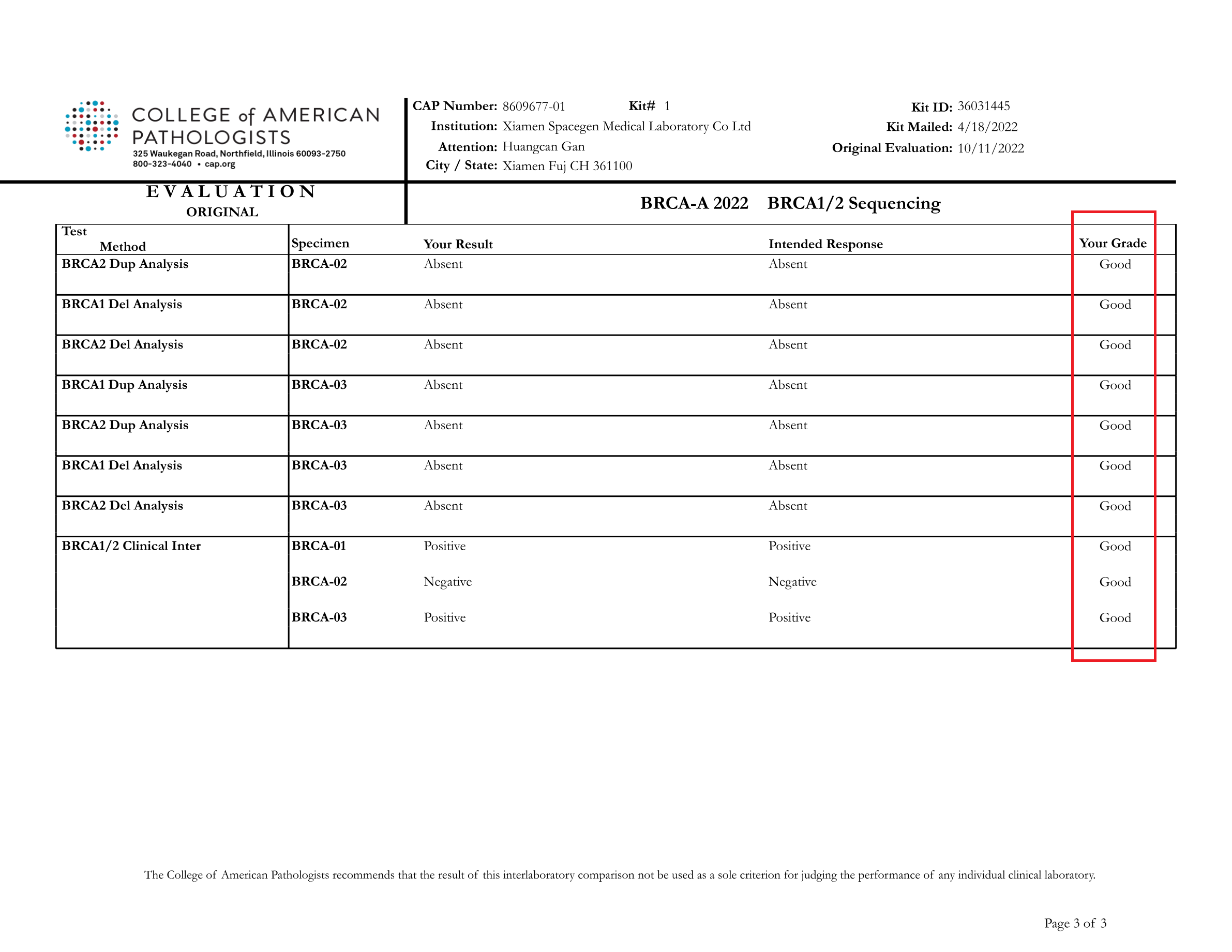
All the results submitted by SpaceSeq MedLab were in full agreement with the official CAP standard results ,proving the accuracy and standardization of our BRCA1/2 gene testing and variant interpretation.
The BRCA-A 2022 external quality assessment is a proficiency testing program jointly organized by CAP and the American College of Medical Genetics and Genomics (ACMG) in 2022 to assess the level of BRCA gene variant detection and clinical annotation capabilities. The external quality assessment included 3 samples, and the assessment was comprehensive, covering the detection of various mutation types such as point mutations, insertions and deletions, and assessed the standardization and accuracy of each laboratory in software bioinformatics analysis and report interpretation.
Breast cancer susceptibility gene (BRCA) is an important oncogene whose coding product is involved in homologous recombination repair of DNA damage.BRCA1/2 gene testing is important in genetic risk assessment, treatment selection, prognosis determination of ovarian cancer, breast cancer, pancreatic cancer, prostate cancer and other related tumors.
As a leading molecular diagnostic company in China, The Project of SpaceSeq ’s BRCA genetic test is of proven quality and has several years of experience in sample data management, which can help early intervention and risk management for high-risk populations.