Current location: Home > NEWS > Industry news
NEWS
PRODUCTS
Multiple cancers patients carried HER2 gene mutations, and HER2-targeted therapy combined with chemotherapy is like "navigation" to snipe cancer cells
News source: Release time:[2022-10-17]

——This article is intended to provide scientific information to medical professionals only
NO.1
What is HER2 gene
The human epidermal growth factor receptor (HER) receptor family plays a central role in the pathogenesis of many cancers. They regulate cell growth, proliferation and differentiation through multiple signaling pathways. All four HER receptors include a cysteine-rich extracellular ligand binding site, a transmembrane lipophilic fragment, and an intracellular structural domain with catalytic activity of a complex kinase. HER2 receptor is a 1255 amino acid, 185 KD transmembrane glycoprotein. HER2 has no known direct activating ligand and may be constitutively activated or become active upon heterodimerization with other family members such as HER1 or HER3. HER2 amplification or overexpression occurs in approximately 15-30% of breast cancers and 10-30% of gastric/gastroesophageal cancers and can be used as a predictive biomarker for prognosis. Overexpression of HER2 is also seen in other cancer types such as ovarian, endometrial, bladder, and lung cancers.
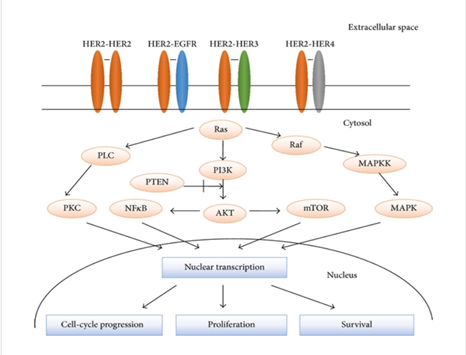
Major transduction-regulated pathways of HER family members [1]
NO.2 HER2 in breast cancer
Most studies on HER2 have been performed in breast cancer and found to induce breast cancer. HER2 is overexpressed in 15-30% of invasive breast cancers and has prognostic and predictive significance. Breast cancers can have up to 25-50 copies of the HER2 gene and up to 40-100 times increase in HER2 protein. HER2 gene amplification is associated with shorter disease-free survival and overall survival in breast cancer. HER2 amplification is also significantly associated with pathological stage of disease, number of axillary lymph nodes in the tumor, histological type, and deletion of estrogen receptor (ER) and progesterone receptor (PR). Evidence suggests that HER2 amplification is an early event in human breast tumorigenesis, with HER2 amplification seen in almost half of ductal carcinomas in situ. HER2-amplified breast cancers have increased sensitivity to certain cytotoxic chemotherapeutic agents and resistance to certain hormonal agents, as well as an increased propensity to metastasize to the brain.
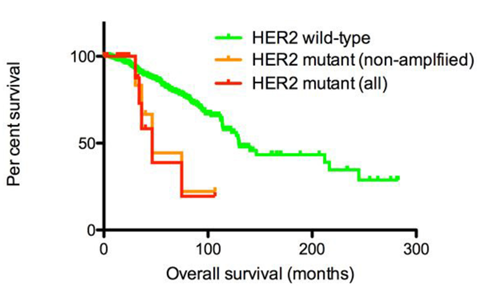
Survival rates of patients with invasive breast cancer stratified by HER2 mutation status [2]
Neoadjuvant chemotherapy or preoperative systemic therapy was initially used for patients with inoperable breast cancer. Several clinical trials have demonstrated that neoadjuvant therapy increases the proportion of patients who are suitable for breast-conserving surgery and shows the same survival benefit compared to adjuvant therapy. In addition, neoadjuvant therapy provides information about the response of the tumor in the organism. Several trials and meta-analyses have shown a strong correlation between pathologic response and prognosis after neoadjuvant therapy. Patients who achieve pathologic complete remission (pCR) after neoadjuvant therapy show significantly better event-free survival than patients with residual disease, especially in HER2-positive and triple-negative breast cancer patients [3].
NO.3
HER2 in gastric cancer
In a study of 166 gastric cancer patients, HER2 overexpression was observed most commonly in gastroesophageal junction (GEJ) tumors and tumors with an intestinal histology. Other studies have also confirmed a high rate of HER2 positivity in GEJ tumors and intestinal subtypes. HER2 overexpression is directly associated with poor prognosis in gastric cancer. In a study of 260 gastric cancers, HER2 overexpression was an independent negative prognostic factor, and HER2 staining intensity was associated with tumor size, plasma membrane infiltration, and lymph node metastasis. Other studies have also confirmed the negative impact of HER2 overexpression in gastric cancer.
NO.4
HER2 in esophageal cancer
HER2 was overexpressed in 0-83% of esophageal cancers, with a higher rate of positivity in adenocarcinoma (10-83%) than in squamous cell carcinoma (0-56%). In a study of 713 patients with surgically resected esophageal adenocarcinoma (EAC), HER2 positivity was found in 17% of patients and was significantly associated with lower tumor grade, lower invasiveness, fewer malignant lymph nodes and the presence of adjacent Barrett's esophagus (BE). A study found that HER2 heterogeneity in HER2-amplified EACs was an independent predictor of worse cancer-specific survival. In addition to EAC, HER2 overexpression was also found to be a negative predictor of survival in esophageal squamous cell carcinoma.
NO.5
HER2 in ovarian cancer
Overexpression of HER2 was seen in 20-30% of ovarian cancer patients. The association between HER2 overexpression and low survival in advanced epithelial ovarian cancer was also confirmed. In a cohort of 73 ovarian cancer patients, patients with HER2 overexpression had significantly poorer survival compared to those with normal expression. In addition, patients with tumors high in HER2 expression were significantly less likely to respond completely to initial treatment or to have a negative re-dissection when preoperative serum CA 125 levels were normal. Although HER2 overexpression has been found to be associated with poorer survival, the effectiveness of HER2 directed therapy remains limited by the low frequency of strong HER2 expression.
NO.6
HER2 in endometrial cancer
In endometrial plasmacytoma, HER2 overexpression has been reported to range from 14% to 80% and HER2 amplification (by fluorescence in situ hybridization) from 21% to 47%. The reported ranges of HER2 overexpression and amplification in endometrioid carcinoma are 1% to 47% and 0% to 38%, respectively. Both HER2 overexpression and amplification are associated with poor prognosis in endometrial carcinoma. Patients with HER2 amplified endometrial plasmacytoma have a significantly shorter overall survival than those without amplification. In addition, patients with high HER2 copy number (ratio > 2.5) performed worse than those with lower HER2 amplification (ratio 2.0-2.5).
NO.7
HER2 in other cancers
The overexpression rate of HER2 in lung cancer has been reported to be about 20%. In addition to overexpression, mutations in HER2 have been reported to be present in lung adenocarcinoma. Mutations are targeted to never or light smokers, Oriental races and females. In invasive uroepithelial bladder cancer, amplification and/or overexpression ranged from 23% to 80% overexpression and 0% to 32% amplification.
NO.8
Targeted therapy for HER2
Trastuzumab is a monoclonal antibody that binds to structural domain IV of the extracellular segment of HER2 receptor. The proposed mechanism of action of trastuzumab includes (1) inhibition of HER2 shedding, (2) inhibition of the PI3K-AKT pathway, (3) attenuation of cell signaling, (4) antibody-dependent cytotoxicity, and (5) inhibition of tumor angiogenesis. Trastuzumab is approved as part of a regimen containing adriamycin, cyclophosphamide, and paclitaxel for the adjuvant treatment of women with lymph node-positive, HER2 overexpressing breast cancer. The approval was based on evidence of significantly longer disease-free survival in women receiving trastuzumab and chemotherapy compared to women receiving chemotherapy alone. Related trials have shown that the use of trastuzumab increases disease-free survival by approximately 50% and overall survival by approximately 33%, regardless of the chemotherapy regimen or dosing sequence of trastuzumab. In metastatic HER2 breast cancer, trastuzumab is also recommended as first-line therapy. In a phase III trial, trastuzumab combined with chemotherapy significantly improved time to disease progression, objective remission rates and 1-year survival compared to chemotherapy alone.
Lapatinib is an orally active dual tyrosine kinase inhibitor that blocks the HER2 and epidermal growth factor receptor (EGFR) pathways. Lapatinib is approved in combination with capecitabine for the treatment of patients with advanced and metastatic breast cancer with HER2 overexpression who have been previously treated with anthracyclines, paclitaxel and trastuzumab. This was based on a study showing a delay in disease progression when lapatinib was used in combination with capecitabine. The risk of disease progression was reduced by 51% and combination therapy was not associated with an increase in toxic side effects.
Lapatinib is also approved in combination with letrozole for the treatment of hormone receptor and HER2 receptor-positive postmenopausal metastatic breast cancer. Letrozole in combination with lapatinib was well tolerated, with significantly higher progression-free survival, overall remission rates and clinical benefit rates compared to letrozole alone.
Pertuzumab is a humanized monoclonal antibody that blocks the activation of the HER2 receptor by blocking dimerization. Pertuzumab triggers action at a different ligand binding site than trastuzumab. It is approved in combination with trastuzumab and docetaxel for patients with HER2-positive metastatic breast cancer who have not previously received hormonal therapy or chemotherapy. Pertuzumab's approval was based on the results of a clinical evaluation of the Pertuzumab and Trastuzumab trials. The trial compared the efficacy of first-line trastuzumab plus docetaxel (plus placebo) with trastuzumab plus docetaxel plus pertuzumab for the treatment of HER2-positive metastatic breast cancer. The results of the study showed that patients treated with pertuzumab in addition to trastuzumab and docetaxel had an average 6.1 month extension in progression-free survival with little or no increase in cardiotoxic effects. Pertuzumab was also approved in combination with trastuzumab and docetaxel for neoadjuvant therapy in patients with HER2-positive, locally advanced, inflammatory or early-stage breast cancer.
Ado-trastuzumab emtansine is an antibody-drug conjugate consisting of a monoclonal antibody trastuzumab linked to the cytotoxic agent mertansine (DM1). Most patients with HER2-positive metastatic breast cancer eventually develop drug resistance. Ado-trastuzumab provides a novel mechanism to overcome trastuzumab resistance by harnessing the cytotoxic activity of trastuzumab-targeted DM1 on HER2 overexpressing cells. Ado-trastuzumab is approved as a single agent for the treatment of patients with HER2-positive metastatic breast cancer. The approval was based on the results of the EMILIA trial, which compared ado-trastuzumab to lapatinib plus capecitabine. The study showed significantly longer progression-free survival and overall survival with lower toxicity compared to lapatinib plus capecitabine.
Neratinib is an oral irreversible inhibitor of HER2 and EGFR tyrosine kinases. A phase II open study in locally advanced breast cancer (LABC) showed a 16-week progression-free survival rate of 75% in 36 patients receiving trastuzumab for the first time, compared to 51% in previously treated patients. Phase III evaluation of neratinib is ongoing in adjuvant trastuzumab-pretreated early-stage breast cancer.
Afatinib is an oral irreversible inhibitor that acts on EGFR/HER1, HER2, and HER4. Results of a phase II study after afatinib treatment of HER2-positive MBC-progressive trastuzumab showed partial remission in 4 of 35 evaluable patients [4].
HER2 has been used as a prognostic and predictive biomarker for breast and gastric cancers, and therapies targeting HER2 have revolutionized the treatment of HER2 overexpressed breast and gastric cancers and improved clinical outcomes. A variety of novel HER2-targeted agents, alone or in combination, are being investigated, and we look forward to more new impacts of HER2-targeted therapies in the near future!
Reference
[1] Mol Biol Int. 2014;2014:852748
[2] Technol Cancer Res Treat. 2021 Jan-Dec;20:1533033820983298
[3] Chin Clin Oncol. 2020 Jun;9(3):32
[4] Biomed Rep. 2013 Jul;1(4):499-505