Current location: Home > NEWS > Corporate news
NEWS
PRODUCTS
Spacegen launched Thyroid Cancer Gene Mutation Detection Kit
News source: Release time:[2022-07-20]
The diagnostic methods of thyroid cancer include laboratory diagnosis, imaging diagnosis and pathological diagnosis. A clear diagnosis is the premise of tumor treatment. Ultrasound-guided fine-needle aspiration biopsy (US/UG-FNAB) is the preferred method for screening benign and malignant thyroid nodules. FNA cytology can provide a definitive diagnosis of benign or malignant nodules in 70–75% of cases, and the remaining 25–30% fall into one of the three categories of indeterminate cytology defined by the Bethesda system. Gene testing for indeterminate types of US-FNA cytology can assist in the diagnosis of benign and malignant thyroid nodules and the classification of thyroid cancer subtypes .
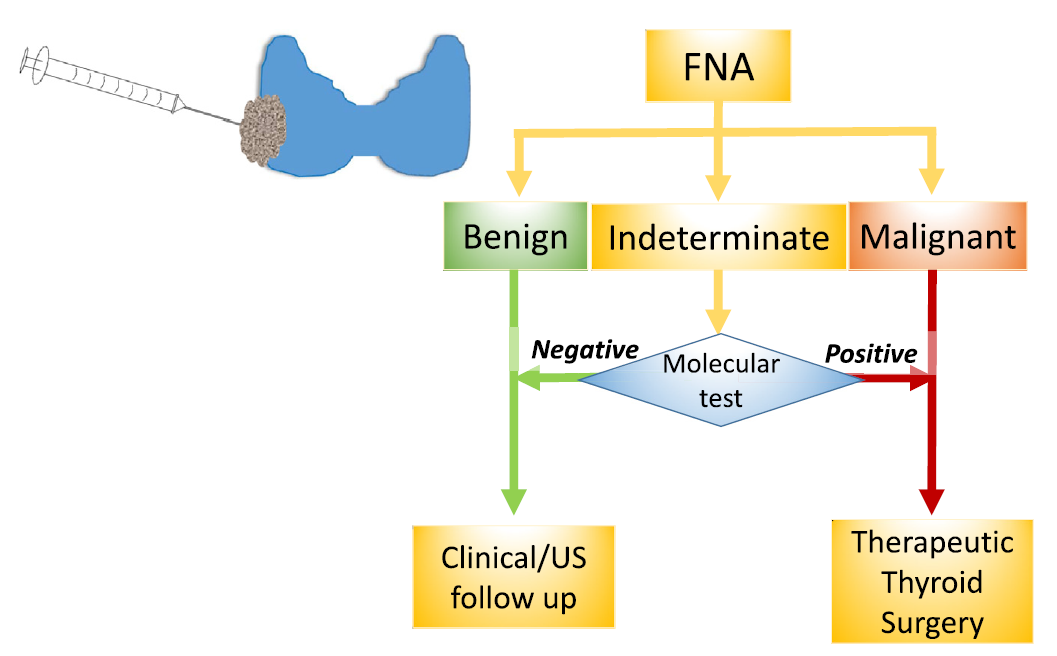
▲Applications of Molecular Diagnostics
Spacegen's thyroid cancer genetic testing panel was launched, it has received great attention from the industry, and many patients have already experienced our testing services. In order to expand the benefited audience, Spacegen has launched a Thyroid Cancer Gene Mutation Detection Kit introduces the detection of the kit Content and application highlights.
Product Introduction
NEW PROUCT LAUNCH
Thyroid Cancer Gene Mutation Detection Kit
Product name: Human Thyroid Cancer Gene Variation Detection Kit (CE-IVD)
Specifications: 16 or 32 tests/kit
Detection item: Consistent with the thyroid cancer gene test submitted for inspection, it includes point mutations and indels of 16 genes, 209 fusion forms of 87 genes, and the expression of 8 internal control genes.
Detection technology: RingCap®+NGS technology
Compatible platform: Illumina platform, including Miseq, NextSeq500/550, Miniseq etc.
Nucleic acid type: DNA+RNA
Recommended sequencing depth: 5000X, internal control gene 2000X
Detection Sensitivity: Detects gene mutations as low as 1% in 5ng FNA samples or 25ng tissue samples, and gene fusions as low as 200 copies/µl in RNA samples.
For people
1. Patients with thyroid nodules with inconclusive results diagnosed by FNA cytology
This is the main application of this kit. According to the 2015 American Thyroid Association (ATA) guidelines for the management of thyroid nodules and differentiated thyroid cancer (DTC) in adults, thyroid nodule FNA cytology reports should be reported using the diagnostic panel in the Bethesda System for Reporting Thyroid Cytopathology. Molecular testing is recommended for patients with indeterminate cytology results, ranging from type III atypical or follicular lesions of undetermined significance (AUS/FLUS), type IV follicular neoplasms/suspected follicular neoplasms (FN/SFN) and Type V Suspected Malignancy (SUSP) . NCCN guidelines and CSCO guidelines also recommend molecular diagnosis in patients with Bethesda type III or IV .

▲NCCN Guideline
It is generally recommended that after puncture, 2-3 needles of FNA samples should be taken and placed in RNA later, and stored at -20°C; genetic testing should be performed when the cytology result is Bethesda type III or IV. However, in reality, due to the difficulty of sample preservation, some physicians choose to perform cytology and genetic testing at the same time. For non-type III and IV cytology results, the genetic test results can be used as a reference. If it is inconsistent with the cytology results, the cytology results shall prevail.
Patients who have been tested for BRAF V600E, no matter whether the test result is negative or positive, can also be added the detection of this kit. For BRAF V600E-negative patients, detection of this panel can improve the detection rate and be used to find other driver genes. For patients with BRAF V600E positive, the detection of this panel can detect whether there are co-mutations of TERT promoter, TP53, PIK3CA, AKT1 and other genes. Co-mutation is a more specific marker of poor prognosis .
In addition, primary non-epithelial tumors (such as thyroid lymphoma, thyroid sarcoma, etc.) and metastatic cancer are not applicable to thyroid cancer standard, so this kit is not applicable.
2. Patients with advanced thyroid cancer who have undergone surgery to choose targeted therapy
The FDA has approved a number of targeted drugs for the systemic treatment of different types of thyroid cancer . The NCCN guidelines also recommend the use of targeted therapy for relapsed and refractory DTC, medullary thyroid carcinoma (MTC), and anaplastic thyroid carcinoma (ATC) . Although the NMPA-approved targeted drugs for thyroid cancer are limited, only Pralsetinib (RET inhibitor, approved for NSCLC indications) and larotrectinib (NTRK inhibitor, approved for pan-cancer indications) require companion diagnostics , the CSCO guidelines also pointed out that “for advanced, aggressive, life-threatening tumors (unresectable recurrent/persistent lesions, soft tissue, bone, and CNS metastases), genomic testing is recommended. Gene mutations of significance" .
For these patients, fresh surgical tissue is recommended to be used as detection sample. FFPE samples can also be used, but it has also been reported that the detection results of FFPE samples may be distorted.
Patients who have been tested for BRAF V600E, no matter whether the test result is negative or positive, can also be added the detection of this panel. Since most of the targeted drugs for thyroid cancer are directed against fusion proteins , for patients with negative BRAF V600E, this kit is currently the most comprehensive panel for detecting gene fusion in China, which can expand the benefit population of targeted drugs. For patients with BRAF V600E-positive DTC or ATC, dabrafenib combined with trametinib can be considered, but this therapy has not been approved in China. The level of evidence may be higher for larotrectinib (NMPA approved) and pratinib (CSCO level II expert recommendation) .
3. Patients with an inherited MTC background and their families
Both guidelines and consensuses recommend genetic testing and screening for people with hereditary MTC background to guide early diagnosis, preventive thyroidectomy and the selection of targeted drugs.
Sample Requirements
2~3 needles of FNA samples, 100 μl RNA for later storage;
Or the size of mung bean grains of fresh tissue from surgery, RNA stored later;
Or rough needle aspiration samples (for cytological diagnosis of Bethesda class V or VI, considering malignant lymphoma, metastatic cancer, or lesions that cannot be clearly classified and require immunohistochemical methods for diagnosis [6]), RNA storage later;
Or wax block/section samples should be determined to contain at least 30% tumor tissue and 5-10 white slices of 5-10 μm.
Inherited MTC germline detection using 5 ml EDTA anticoagulation.
RNA later can store samples for 1 week at room temperature, 1 day at 37°C, 1 month at 4°C, and long-term storage at -20°C or -80°C.
In addition to the requirements for samples, this kit has corresponding quality control standards for the total amount and integrity of the extracted nucleic acid, the amount and fragment size of the library, the sequencing depth, homogeneity and target sequence ratio of the offline data, etc. .
Conclusion
Spacegen's Thyroid Cancer Gene Mutation Detection Kit contains all the clinically significant genes recommended in the guidelines and expert consensus. The kit has high sensitivity, simple operation, and sets strict quality control standards in multiple links such as nucleic acid extraction, library preparation, and off-machine data.
Spacegen is committed to providing the most innovative products and services for individualized precision medical testing of tumors. Please contact us for more information by spacegen@ispacegen.com