Current location: Home > NEWS > Industry news
NEWS
PRODUCTS
Analysis of resistance to osimertinib:The first-line status is unshakable, the multi-drug resistance mechanism needs to be explored
News source: Release time:[2022-07-01]
Lung cancer is the second most common malignant tumor in the world in terms of morbidity and mortality [1,2]. According to the GLOBOCAN analysis report of the global tumor epidemiological statistics in 2020, the number of new cases of lung cancer worldwide reached 2.207 million, ranks as the second after breast cancer; the number of deaths reached 1.796 million, ranked as first among all cancer types. In my country, lung cancer is not only the most common malignant tumor in terms of morbidity and mortality [2,3]. In 2020, the estimated new cases of lung cancer in my country will reach 816,000, accounting for 17.9% of all new cancers, and the number of deaths will reach 715,000, accounting for 23.8% of the total cancer deaths [4]. According to the age-standardized rate (ASR), the incidence of lung cancer in my country in 2020 for men and women will be ASR 47.8/100,000 and 22.8/100,000, , and the mortality rate will be ASR 41.8/100,000 and 19.7/100,000 [4] .

Lung cancer can be divided into two histopathological types, non-small cell lung cancer (NSCLC) and small cell lung cancer, of which NSCLC is the most common, accounting for 85% of all lung cancers [5]. For driver gene-negative advanced NSCLC, the median progression free survival (mPFS) of traditional platinum-based doublet chemotherapy is only 4 months to 6 months, and the median overall survival (mOS) is only 4 months to 6 months. 10 months - 12 months. [6 -9]
Tyrosine kinase inhibitors (TKIs) can bring significant clinical benefits to patients with EGFR-mutant non-small cell lung cancer (NSCLC), changing the treatment landscape for EGFR-mutant lung cancer


1 Mechanisms of resistance to osimertinib
A precision medicine approach has been successfully applied in medical oncology to treat non-small cell lung cancer (NSCLC) by identifying targeted driver molecular aberrations; mutations that activate epidermal growth factor receptor (EGFR) are the most common. Osimertinib is a third-generation, wild-type-retained, irreversible EGFR tyrosine kinase inhibitor (TKI) that has been shown to progress to first- and second-generation EGFR-TKIs when the T790M resistance mutation was identified. After that, it initially showed significant activity.
After that, in the FLAURA trial, a standard of care based on the overall survival benefit of the first-generation TKIs erlotinib and gefitinib was reported. For patients who remain on osimertinib, identification of resistance mechanisms is critical for the development of new targeted therapies. In addition, innovative drugs or combination therapies are currently being developed for cases in which specific resistance mechanisms cannot be identified.
In this review, we analyze post-osimertinib treatment options for EGFR-mutant NSCLC and provide an outlook on ongoing clinical trials. A guideline is proposed, reported in the FLAURA trial, based on the overall survival benefit of the first-generation TKIs erlotinib and gefitinib on the standard of care. For patients who remain on osimertinib, identification of resistance mechanisms is critical for the development of new targeted therapies. In addition, innovative drugs or combination therapies are currently being developed for cases in which specific resistance mechanisms cannot be identified.
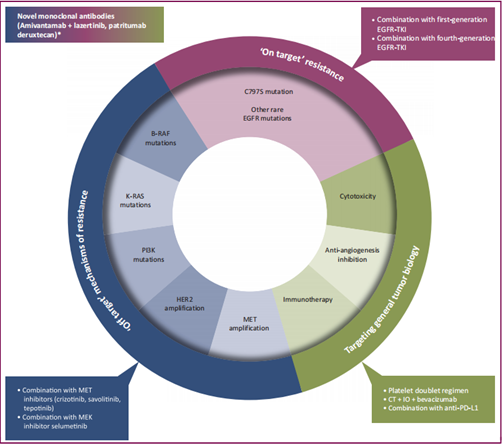
▲Resistance mechanisms to osimertinib and potential therapeutic strategies to overcome resistance [10]
Whether it is first-line or second-line use of osimertinib, resistance will inevitably occur.
2 Osimertinib treatment strategy [12]
3 Proposed approach to treatment of NSCLC progression with osimertinib
If progression to osimertinib is clinically suspected, complete imaging of the chest, abdomen, pelvis, and central nervous system is recommended. When progression is asymptomatic and the disease is slowly increasing, continuation of osimertinib is a reasonable option in addition to rigorous clinical and radiological monitoring of the disease. Association of LAT with oligoprogressive sites of disease may improve prognosis. In addition, patients with limited CNS progression should continue osimertinib while receiving local therapy (stereotactic radiotherapy or neurosurgery in selected cases). If CNS involvement is extensive and symptomatic, a different systemic therapy with high intracranial penetration (eg, carboplatin/pemetrexed chemotherapy) should be used as an alternative or local therapy (ie, whole brain irradiation).
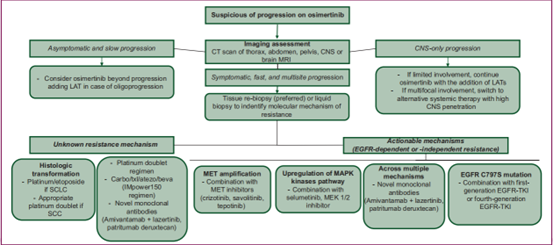
▲Potential strategies for disease progression following osimertinib resistance
4 Other ongoing clinical trials
Additional Phase I/II studies are currently underway to evaluate the safety and clinical activity of new combination approaches to overcome osimertinib resistance.
The ORCHARD trial (NCT03944772) is investigating multiple treatment options for first-line progression of osimertinib, an open-label, biomarker-directed Phase II study with an innovative platform design. Enrolled patients underwent tumor biopsies at disease progression to determine resistance mechanisms and were assigned to appropriate treatment groups based on biomarker analysis. Biomarker-positive patients were assigned to biomarker-matched study treatments in Arm A, including osimertinib in combination with savolitinib for MET amplification, gefitinib for C797X EGFR mutations, necitumumab for EFGR amplification, and others that may be added in the future combination. Patients without biomarkers were assigned to an investigational treatment (durvalumab + platinum chemotherapy, osimertinib + necitumumab, or a further combination) in arm B, arm C was in observation arm, there were some patients who were not eligible in the first two groups and were treated according to Local practice for treatment. The study is a Phase I/II study (NCT03784599) investigating the combination of osimertinib.
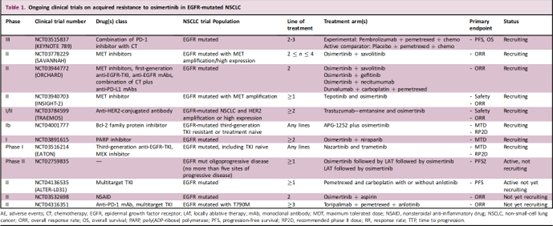
▲Ongoing clinical trial on acquired resistance to osimertinib in EGFR-mutant NSCLC
Reference
[1] Siegel RL, Miller KD, Fuchs HE, et al. Cancer Statistics, 2021. CA Cancer J Clin, 2021, 71(1): 7-33. doi: 10.3322/caac.21654
[2] Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCA N estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin, 2021. doi: 10.3322/ caac.21660
[3] Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin, 2016, 66(2): 115-132. doi: 10.3322/caac.21338
[4] https://gco.iarc.fr/
[5] Rosell R, Karachaliou N. Large-scale screening for somatic mutations in lung cancer. Lancet, 2016,387(10026):1354-1356.doi:10.1016/S0140-6736(15)01125-3
[6] Socinski MA, Bondarenko I, Karaseva NA, et al. Weekly nab-paclitaxel in combination with carboplatin versus solvent-based paclitaxel plus carboplatin as first-line therapy in patients with advanced non-smallcell lung cancer: final results of a phase III trial. J Clin Oncol, 2012, 30(17): 2055-2062. doi: 10.1200/JCO.2011.39.5848
[7] Hayashi H, Okamoto I, Morita S, et al. Postprogression survival for firstline chemotherapy of patients with advanced non-small-cell lung cancer. Ann Oncol, 2012, 23(6): 1537-1541. doi: 10.1093/annonc/mdr487
[8] Scagliotti GV, Parikh P, von Pawel J, et al. Phase III study comparing c i s pl at i n plu s gemc it abi ne w it h c i s pl at i n plu s pemet re xed i n chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol, 2008, 26(21): 3543-3551. doi: 10.1200/jco.2007.15.0375
[9] Paz-Ares LG, de Marinis F, Dediu M, et al. PARAMOUNT: Final overall survival results of the phase III study of maintenance pemetrexed versus placebo immediately after induction treatment with pemetrexed plus cisplatin for advanced nonsquamous non-small-cell lung cancer. J Clin Oncol, 2013, 31(23): 2895-2902. doi: 10.1200/jco.2012.47.1102
[10] Treating disease progression with osimertinib in EGFR-mutated non-smallcell lung cancer: novel targeted agents and combination
[11] Nat Cancer 2, 377–391 (2021)
[12] Front Oncol. 2020 Dec 18;10:602762.