Current location: Home > NEWS > Corporate news
NEWS
PRODUCTS
Authoritative certification! Spacegen Medical Laboratory passed the external quality evaluation of US CAP NGSST-B 2021
News source: Release time:[2022-02-16]
Recently, the results of NGSST-B (Next-Generation Sequencing Solid Tumor) organized by the American Association of Pathologists (CAP) announced that Spacegen Medical Laboratory have passed the evaluation with zero defects, marking the level of high-throughput sequencing (NGS) detection and analysis of Spacegen, and the quality management system has reached the world-class level!
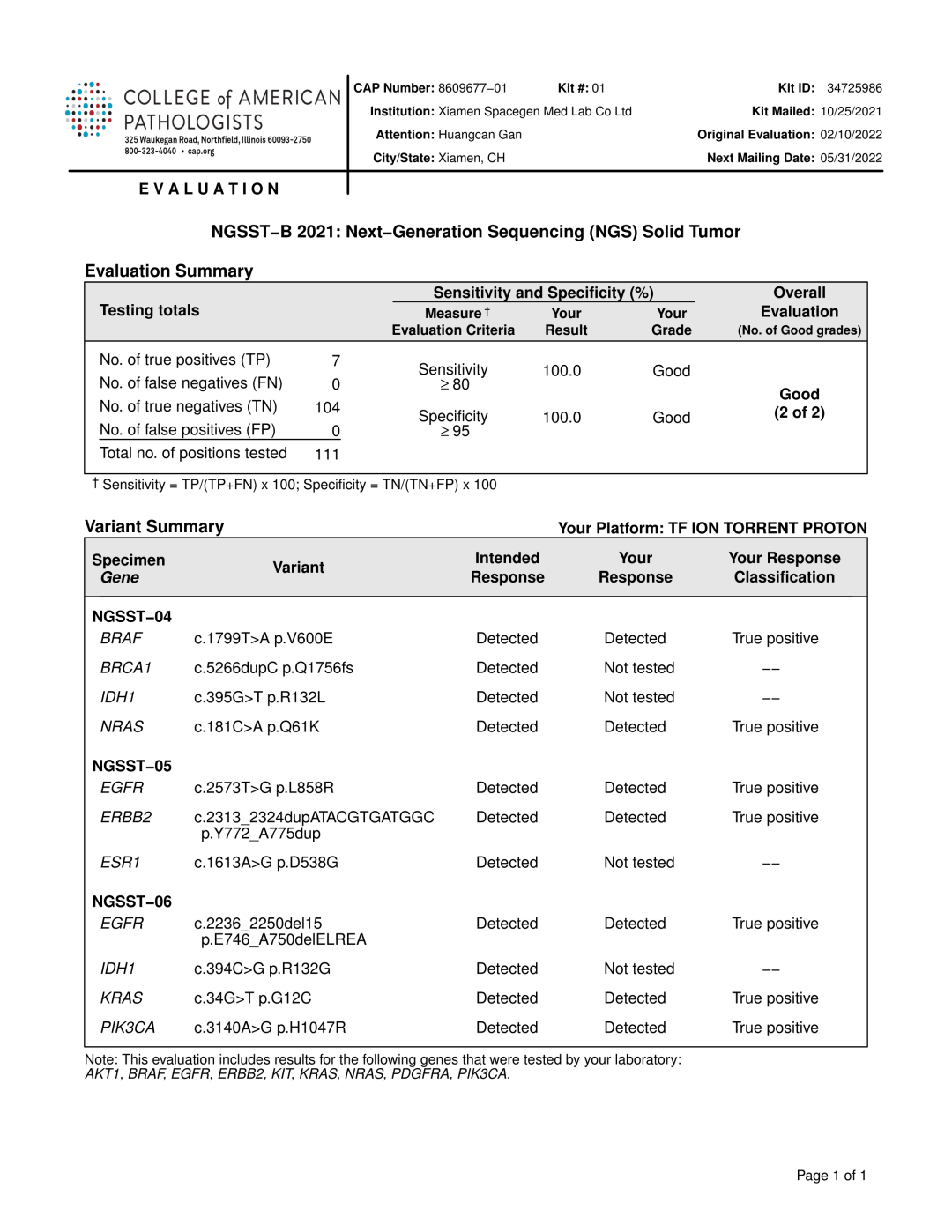
NGSST(Next-Generation Sequencing Solid Tumor)is a PT project newly launched by CAP in 2016 to evaluate the ability of global gene diagnosis laboratories to detect gene variation in solid tumor tissues using NGS.
A total of three samples were tested in this project. The sample numbers are n NGSST-04, NGSST-05, NGSST-06 respectively. The assessment indicators include AKT1, ALK, BRAF, BRCA1, EGFR, erbB2, IDH1, kit, KRAS, NRAS, PIK3CA and other tumor related genes.
In the NGSST capability verification project, the test results of Spacegen Medical Laboratory are completely consistent with the standard results published by CAP. On the one hand, it is verified that Spacegen, as a leading molecular diagnosis enterprise in China, has fully recognized and affirmed the standardization and standardization of the detection quality system. It is also verified that the detection level of the accuracy and stability of Spacegen Medical Laboratory has reached the world top level.
For a long time, Spacegen’s intensive cultivation high-throughput sequencing technology has continued to focus on the field of gene technology services, and is committed to becoming an international leading professional molecular diagnosis company to continuously protect human health.
About CAP
Cap (College of American Pathologists, CAP) is the American Association of pathologists. As the most authoritative organization in the field of Pathology, it is also considered to be one of the most authoritative clinical testing laboratory accreditation institutions in the world. The CAP certification is also an international laboratory standard (gold standard) recognized by the world as suitable for medical laboratories.
The certification ensures that the laboratory meets the quality standards through strict requirements, so as to improve the actual work of the laboratory and ensure the accuracy of test results. The clinical laboratory certified by CAP means that its laboratory testing level has reached the world top level and has been recognized by relevant international institutions.
XIAMEN SPACEGEN CO.,LTD.
Benefit public health
XIAMEN SPACEGEN CO.,LTD., founded in 2015, is a national high-tech enterprise with leading entrepreneurial talents settled in Xiamen "double hundred plan". It integrates R & D, production, sales and services, and is committed to providing products and services for cancer screening, disease diagnosis, individualized drug selection, efficacy recurrence monitoring and accurate medical testing. Equipped with more than 3500 ㎡ of international standard GMP workshop and more than 2000 ㎡of R & D quality control laboratory, it has first-class advanced facilities and equipment, has established a sound quality management system, and has passed the TUV ISO13485 medical device quality management system certification in South Germany. Xiamen Spacegen Medical Laboratory, a wholly-owned subsidiary of XIAMEN SPACEGEN CO.,LTD., carries out detection projects on the technology platforms of high-throughput sequencing (NGS), fluorescence quantitative PCR, digital PCR and nucleic acid mass spectrometry to provide high-quality and low-cost accurate medical gene detection services for medical and health institutions and the public.