Current location: Home > NEWS > Industry news
NEWS
PRODUCTS
"Biological missile" ADC drugs are in the ascendant, opening up a new situation in tumor treatment
News source: Release time:[2021-12-06]
The concept of ADC drugs has a long history. It wasfirst proposed by Paul Ehrlich in 1900. However, in the past, it was limited bythe high technical threshold required for its synthesis, long-term off-targetand specific antigen discovery and other technical problems. The ADC industryhas experienced its ups and downs. Scientists have conducted many years oftrials and continuous iterations on how to synthesize ADC drugs, improvesafety, reduce off-target and toxic side effects, but ADC drugs have not usheredin rapid development until recent years.
01
Antibody Drug Conjugate,ADC
The main components of antibody conjugate drugsinclude antibodies, linkers and small molecule cytotoxic drugs(small molecular cytotoxic drug,SM).The monoclonal antibody andthe toxic drug molecule are coupled together through the linker (specificlinker), the specific targeting of the antibody is used to transport the drugmolecule to the target tissue. Some antibodies also have anti-tumor pharmacodynamiceffects, such as In Kadcyla, trastuzumab (ado-trastuzumab) and maytansine havea synergistic effect.
Therefore, in the field of tumor therapy, ADC not onlyhave high selectivity of targeting, but also have the powerful lethality ofchemotherapy, which is called "biological missile."
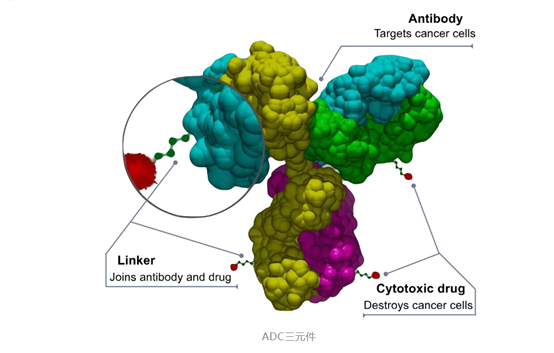
The antibody is responsible for finding tumor cells,and the linker allows the antibody to carry out entrainment, bringing smalltoxic molecules into the tumor cells, and the small molecules are a sharp swordto kill the tumor cells.
In addition, there are also antibodies that haveanti-tumor efficacy. Due to the large differences in the design of differentADC drugs, even for different drugs with the same target, the differences inrecognition sites, connection sites, linkers, and the small connected moleculeswill cause large differences in drug toxicity.
02
The development of ADC
ADC has experienced threegenerations of technological changes, and the treatment has been greatlyimproved.
The small molecules of thefirst-generation ADCs were not toxic enough, and not stable enough, most ofthem ended in failure
The second generation of the ADC drugs uses more toxicmolecules to overcome the weakness of the first generation.
The birth of the third-generation ADC drugs is mainlydue to the development of Bioconjugate techniques.
Pfizer withdrew Mylotarg from the market in 2010 dueto serious fatal liver damage. After the withdrawal, Pfizer made a series ofadjustments to it, especially the optimization of the drug delivery method, andcome back in 2017.
The second-generation of ADC drugs represented byAdcetris and Kadcyla: In 2019, the sales of Seagen's Adcetris and Roche'sKadcyla both exceeded the $1 billion, and have high growth potential in thefuture, have gradually grown into a "blockbuster drug"
Enhertu and Proselyte are the representatives of thethird-generation of ADC drugs. The Enhertu indication approved in December 2019was HER2-positive breast cancer. Due to its large patient population, themarket prospects are huge. Trodelvy is the world's first TROP-2 targetedantibody-drug conjugate therapy, and it is also the first drug to be marketedin the 37 years since the establishment of Immunomedics. It reached a net saleof US$20.1 million in the first two months after its launch.
ADC drugs have experienced the "bumpydevelopment" of launching, withdrawal and remarketing from the firstproduct Mylotarg, however the ADC drugs have ushered in a concentrated harvestperiod in the past five years;
Drug Name | Target | Coupling Enzyme | Company | Indication | Approved Date |
Mylotarg | CD33 | calicheamicin | Pfizer | CD-33 positive leukemia(AML) | 2000(2001withdrew ) 2007 launching again |
Adcetris | CD30 | MMAE | Seagen | classic Hodgkin's lymphoma,systemic gradient large cell lymphoma | 2011.8 |
Kadcyla | HER2 | DM1 | Roche | Her2 positive breast cancer | 2013.2 |
Besponsa | CD22 | calicheamicin | Pfizer | r/r B cell acutelymphocytic leukemia(HCL) | 2017.8 |
Lumoxiti | CD22 | PE38 | AZ | r/r hairy cell leukemia | 2018.9 |
Polivy | CD79β | MMAE | Roche | r/r DLBCL | 2019.6 |
Padcev | Nectin-4 | MMAE | Seagen/ Astellas | advanced urothelial carcinoma | 2019.12 |
Enhertu | HER2 | Dxd | AZ/Daiichi Sankyo | Her2 positive breastcancer | 2019.12 |
Trodelvy | TROP-2 | SN38 | Immunomedics/Everet medicine | TNBC(3L) | 2020.4 |
Blenerep | BCMA | MMAF | GSK | r/r MM | 2020.8 |
Akalux | EGFR | IRDye700DX | Rakuten Aspyrain | squamous cellcarcinoma of the head and neck | 2020.9 |
Lonca | CD19 | PBD | ADC Therapeutics SA | diffuse large cells | 2020.04 |
Vidicituzumab | Her-2 | MMAE | Remegen | Locally advanced or metastatic gastric cancer | 2020.06 |
Information Sources:FDA、NMPA
03
Introduction of the new XDCtechnology (partial)
Bicycle:Bicyclic peptide conjugateddrugs
The tumor tissue is more permeable, the tumor tissuedrug concentration/plasma drug concentration ratio is significantly higher thanthat of traditional ADC, and the anti-tumor activity is also stronger.
Coherent Biopharma (Suzhou)Co., Ltd.:Dual targeting XDC
Ligand targeting, targeting 2 targets at the same time;
Mersana:Polymer Linker
Coupled with STING agonist, 100 times more active thanfree STING agonist. AF-PHA toxin, DAR=6, the anti-tumor activity is thestrongest.
Syaffix:GlycoConnect
It can be coupled with 1 or 2 toxins, with a doublewarhead design, and the technology is licensed to Miac.
Elucida:Nanoparticle conjugated drugs
Ultra-small nanoparticles (6-7) are simultaneouslycoupled with targeting molecules and toxins. Has better tumor tissuepermeability.
Sanofi:Coupling siRNA, siRNAtargeting RIG-1 and PLK-1, antibody targeting EphA2R. The role of antibodies ismainly to deliver siRNA to a specific location, and can also extend thehalf-life of siRNA molecules.
04
ADC Drug Outlook
Due to the advantages of clear targets, maturetechnology, and good selectivity, antibody-conjugated drug research will stillbe hot in the future. ADC is like a 5G track in communications. Although theADC has developed for decades, there is still huge opportunities forimprovement.
Currently studies have shown that the proportion ofADC compounds delivering effector molecules to target cells is far less than1%, but the targeting drug delivery is still much higher than that oftraditional systemic drug delivery, the incidence of adverse reactions is alsosignificantly lower. It can be seen that ADC still has many shortcomings insome places, but more advantage over traditional drugs. There is a lot of roomfor innovation and exploration in new antibody-conjugated drug technology, andmore clinical verification is still needed.