Current location: Home > NEWS > Industry news
NEWS
PRODUCTS
The rise of pan-cancer new forces, NTRK targeted therapy can be expected in the future
News source: Release time:[2021-11-30]
The advancement of targetedcancer therapy has confirmed that gene fusion can be used as a targeted type ofgene mutation.The survival rate of targetedtherapy is much higher than that of standard chemotherapy.NTRK fusion is such a targetable gene mutation.

NTRK, which is not limited tocancer types, has become a hot tumor treatment target in recent years. NTRK hasalso been officially written into clinical guidelines for many cancer types.In the NCCN guidelines, NTRK hasbecome the recommended detection gene for solid tumors such as non-small celllung cancer, breast cancer, hepatobiliary tumors, gastric cancer, bowel cancer,and melanoma.
"The source of evil", NTRK isan important site for the onset of multiple cancers
The neurotrophic tyrosinereceptor kinase gene (NTRK) is composed of three types of genes: NTRK1, NTRK2and NTRK3, which encode three membrane receptor proteins: tropomyosin-relatedkinase A (TrkA), tropomyosin-related kinase B (TrkB), and tropomyosin-relatedkinase C (TrkC).
When TRK receptorprotein binds to the corresponding ligand, different physiological functionscan be achieved by activating downstream signal pathways [1]. Whenthe NTRK gene is fused with other genes, the abnormal TrK fusion protein cancontinue to activate multiple downstream signal pathways of the ligand, andpromote the proliferation and metastasis of tumor cells. More than 30 NTRK genefusion types have been found, such as ETV6-NTRK3, NFASCNTRK1, BCAN-NTRK1, etc.[2] NTRK gene fusion exists in a variety of solid tumors. Studies have shownthat NTRK fusion mutations have been found in more than 25 types of tumors[3-5], including thyroid cancer, colon cancer, lung cancer, pancreaticcancer, etc. The overall incidence is about 0.2%. However, its incidence insome rare cancers, such as salivary gland secretory carcinoma (MASC), secretorybreast cancer, pediatric fibromyoma, and congenital renal cancer can exceed 90%[6,7]
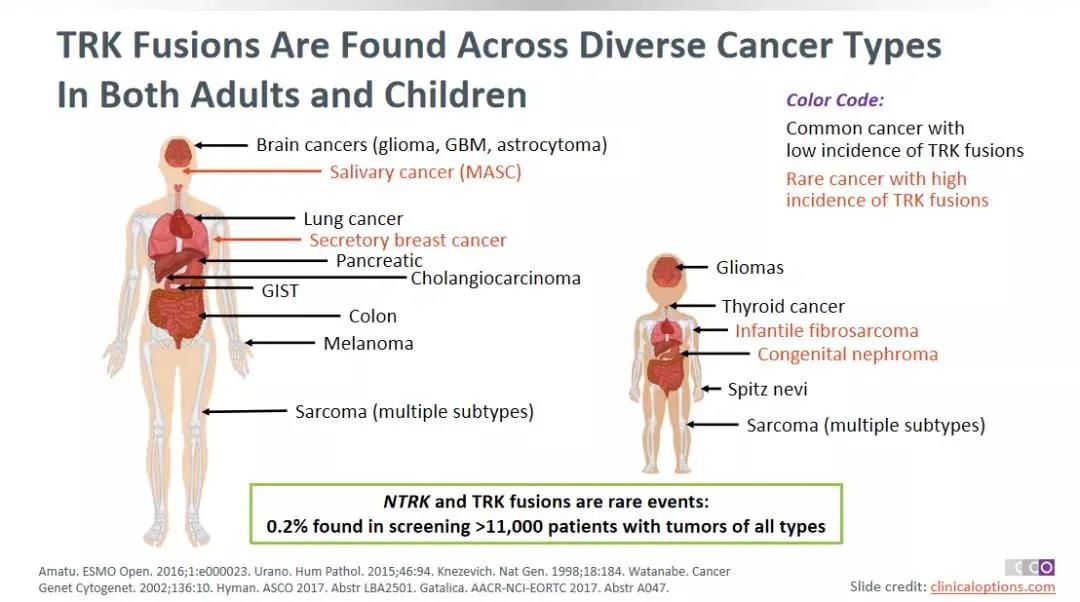
▲The pathogenesis of NTRK in different tumor types [6,7]
NTRK inhibitors are quickly approvedglobally, opening a new era of pan-tumor therapy
In recent years, the globalresearch and development of NTRK targeted drugs has quickly been approved formarketing, making precise treatments a reality.
Among them, Entrectinib(tentative name: Entrectinib) is a TKI drug that targets the fusion of NTRK andROS1 [8]. It was approved by the U.S. Food and Drug Administration (FDA) foraccelerated marketing in August 2019 and was awarded Breakthrough Therapy (BTD)which is used to treat patients with solid tumors carrying NTRK gene fusions.In the same year, the drug was also approved for marketing in Japan and wasawarded as a pioneer drug. In the following year, it passed the priority reviewand obtained the marketing authorization in Europe, becoming anotherblockbuster "broad-spectrum" anti-cancer drug.
According to research and analysis, the overallefficacy of pan-tumor NTRK fusion is acceptable.
For different tumortypes, the curative effect of Entratinib is amazing! Among them, the ORR ofpatients with sarcoma treated by entricinib was 57.7%, and the DoR was 15.0months! Treatment of patients with MASC has an ORR of 83.3% and an immatureDoR. The ORR for NSCLC patients was 63.6% and the DoR was 19.9 months; the ORRfor pancreatic cancer patients was 75% and the DoR was 12.9 months.
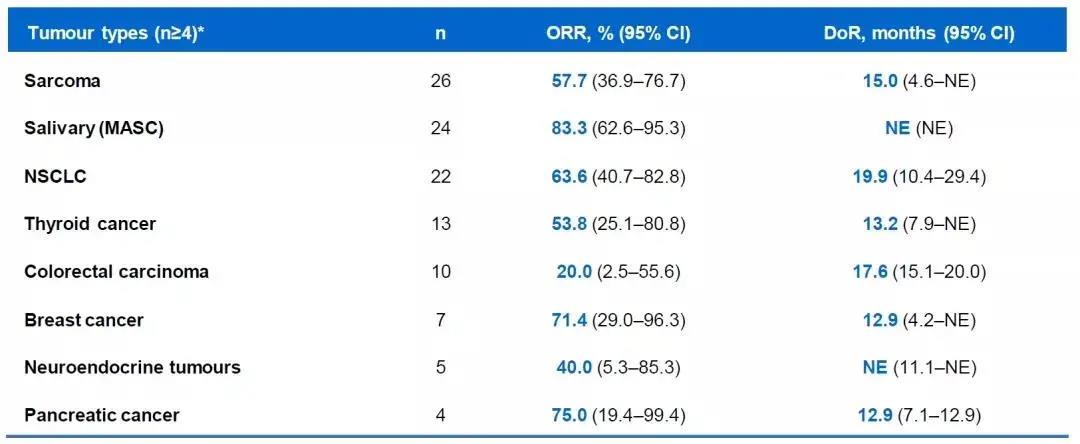
▲The treatment effect of entratinib treatment effect
Satisfactorily, whether it isNSCLC and BC with a higher incidence, a sarcoma that lacks clinically effectivetreatment, or MASC with a higher NTRK mutation rate, a better clinical effectcan be obtained from the treatment of entratinib.
The safety analysisshowed that [2], in the NTRK fusion positive safety assessmentpopulation (n=68), most adverse events were grade 1 and grade 2, and only 7.4%(5/68) of patients had grade 4 adverse events which is acceptable tolerance andcontrollable safety.
NTRK pan-tumor targeteddiagnosis and treatment is written into the guidelines
With the rapid development ofprecision medicine, NTRK has also been officially included in clinicalguidelines for many cancer types, such as NCCN (National Comprehensive CancerNetwork) guidelines for solid tumors such as NSCLC, BC, hepatobiliary tumors,gastric cancer, colorectal cancer, and melanoma. It is recommended to detectNTRK fusion mutations.
Simultaneously, NTRK inhibitors represented by Entratinib arealso recommended by the guidelines as the preferred choice for NTRK fusionsolid tumors.
In China, no TRK inhibitor forpan-solid tumors has been approved for marketing, but NMPA has included Bayer’slarotinib and Roche’s emtricinib on May 26 and October 28, 2021 in the priorityreview. China will soon usher in the first pan-solid tumor targeted drug.
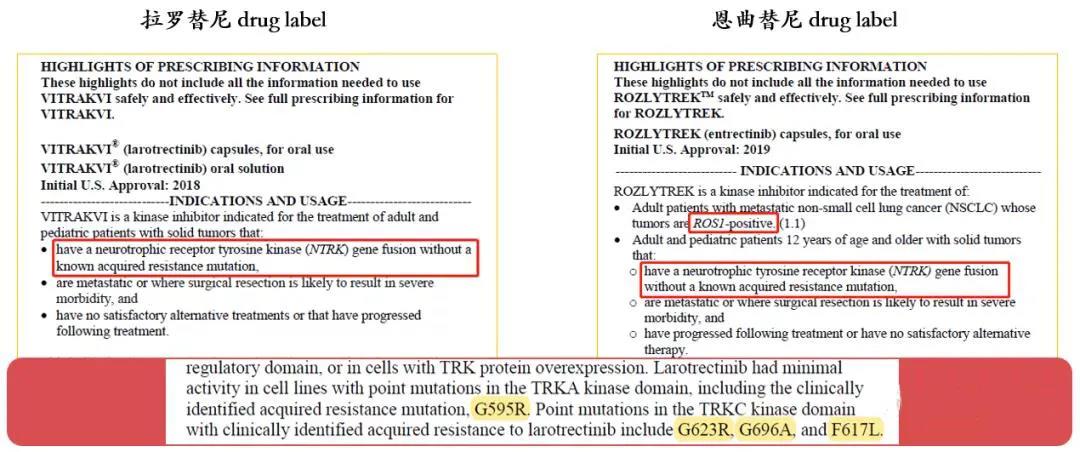
Who should consider to do the NTRKdetection?
1)In most of the time, oncogene mutations are mutually exclusive.The first thing needs to be excluded is the common gene mutations. It isrecommended to do the NTRK gene fusion detection if there are no other drivinggene mutations.
2)NTRK gene fusion detection depends on the type of tumor, it isrecommended to do the NTRK gene fusion detection for high-frequency NTRK fusiontumors (children's tumors and rare tumors, etc.).
3)There is no need to detect NTRK gene fusion if the patients canbe cured by traditional treatments . If the toxicity of the traditionaltreatment is too strong or unavoidable, it is recommended to perform NTRK genefusion detection.
4)Patients who are considered suitable for systemic treatment arealso recommended for NTRK gene fusion detection.
5)When there is no effective systemic treatment method, NTRK genefusion detection is also recommended.
6)Although there are reasons to test the latest samples frompatients who have progressed after treatment, NTRK gene fusion may exist fromthe beginning of the cancer, the initially resected tumor sample is usuallysufficient. Therefore, NTRK gene fusion detection can be performed in theinitial tumor sample.
7)Patients with disease progression after treatment with larotinibor entretinib is recommended to detect NTRK gene to determine drug-resistantmutations. If there are drug-resistant mutations, the use the second-generationNTRK inhibitor LOXO-195 is recommended.
Spacegen also developed HumanNTRK Gene Fusions Detection Kit based on Real Time PCR platform, whichcan provide medication guidance for patients with solid tumor, who are NTRKfusion-positive.
Performance Parameter
Product Name | Detection Technology | Specifications | Compatible Instruments | Sample Types |
Human NTRK Gene Fusions Detection Kit | Real Time PCR | 20tests/kit | Stratagene Mx3000P™, ABI7500 etc. | tumor tissue Slice, pleural effusion |
Detection Advantage
RapidDetection
Only needs one day to finish the detection.3days from samplingto reporting.
Stableand Reliable
Cross tube detection to avoid contamination.
GoodRepeatability
Can be performed in the general PCR Labs. Results with goodreproducibility can be easily obtained without special training.
EasyOperation
Flow-based operation and simple experiment process.
Reference
[1]Fischer H. Entrectinib, a TRK/ROS1 inhibitor with anti-CNStumor activity: differentiation from other inhibitors in its class due to weak interactionwith P-glycoprotein. Neuro Oncol. 2020 Jun 9;22(6):819-829.
[2]Doebele RC,et al. Entrectinib in patients with advanced ormetastatic NTRK fusion-positive solid tumours: integrated analysis of threephase 1-2 trials. Lancet Oncol. 2020 Feb;21(2):271-282.
[3]Vaishnavi A, Le AT, Doebele RC. TRKing down an old oncogenein a new era of targeted therapy. Cancer Discov. 2015;5(1):25-34.
[4]Lange AM, Lo HW. Inhibiting TRK proteins in clinicalcancer therapy. Cancers (Basel). 2018;10(4):105.
[5]Cocco E, Scaltriti M, Drilon A. NTRK fusion-positive cancersand TRK inhibitor therapy. Nat Rev Clin Oncol. 2018;15(12):731-747.
[6]Amatu. ESMO Open. 2016;1:e000023. Urano. Hum Pathol.2015;46:94. Knezevich. Nat Gen. 1998;18:184. Watanabe. Cancer Genet Cytogenet.2002;136:10.
[7] Hyman. ASCO 2017. Abstr LBA2501. Gatalica. AACR-NCI-EORTC2017. Abstr A047.