Current location: Home > NEWS > Corporate news
NEWS
PRODUCTS
Good news! Spacegen has passed the external quality evaluation for national solid tumor somatic mutation based on next generation sequencing
News source: Release time:[2021-11-01]
Recently, the National Center for Clinical Laboratory(NCCL) announced the results of the “Pre-study of the national quality evaluationfor solid tumor somatic mutations based on next generation sequencing in 2021”.The report showed that Spacegen Medical Laboratoryhas passed the quality evaluation with high score. It fully demonstratedthe accuracy and authority of Spacegen’s detection results, marking thatSpacegen Medical Laboratory 's detection capabilities and quality managementsystem have been recognized by national authoritative institutions.
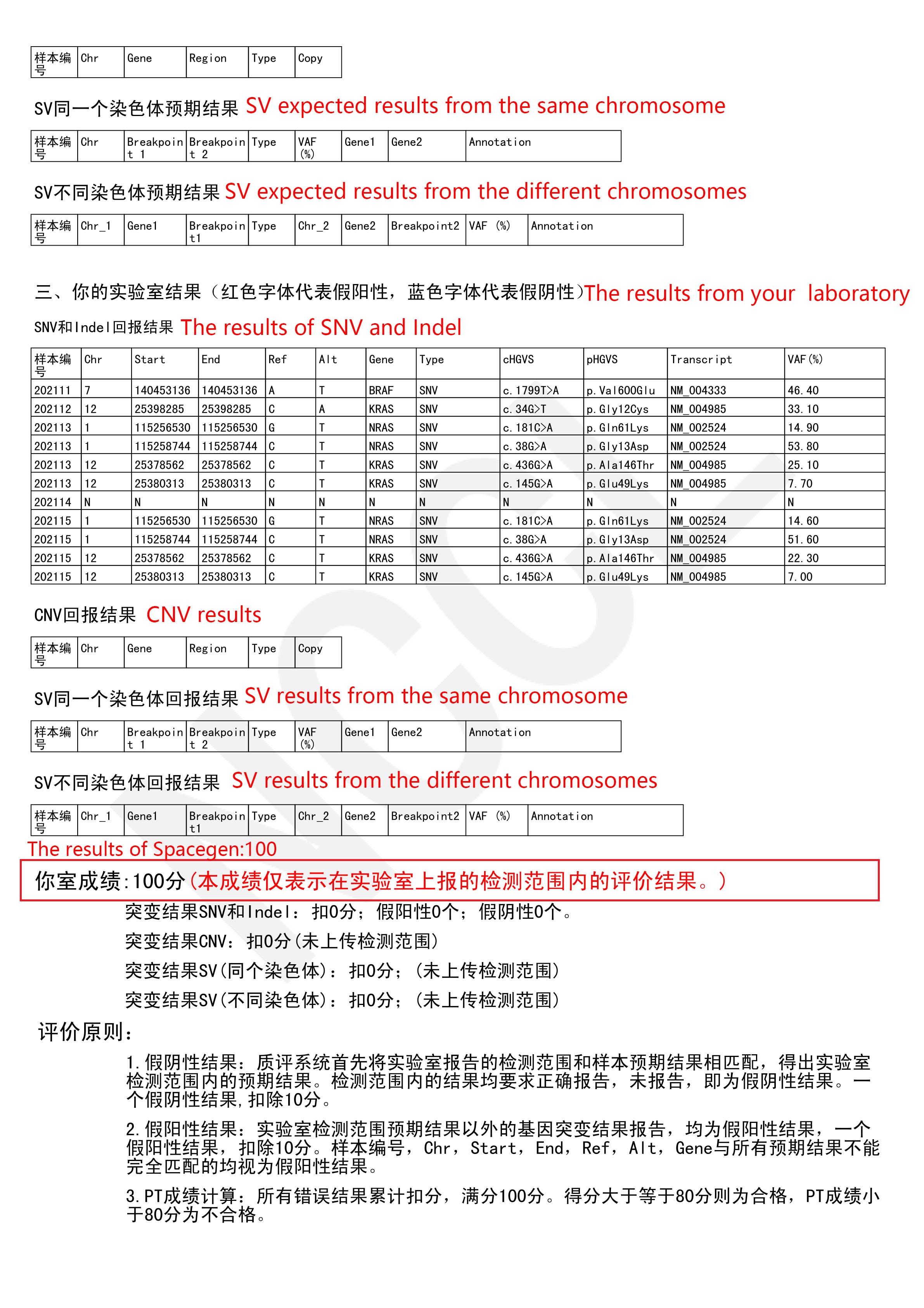
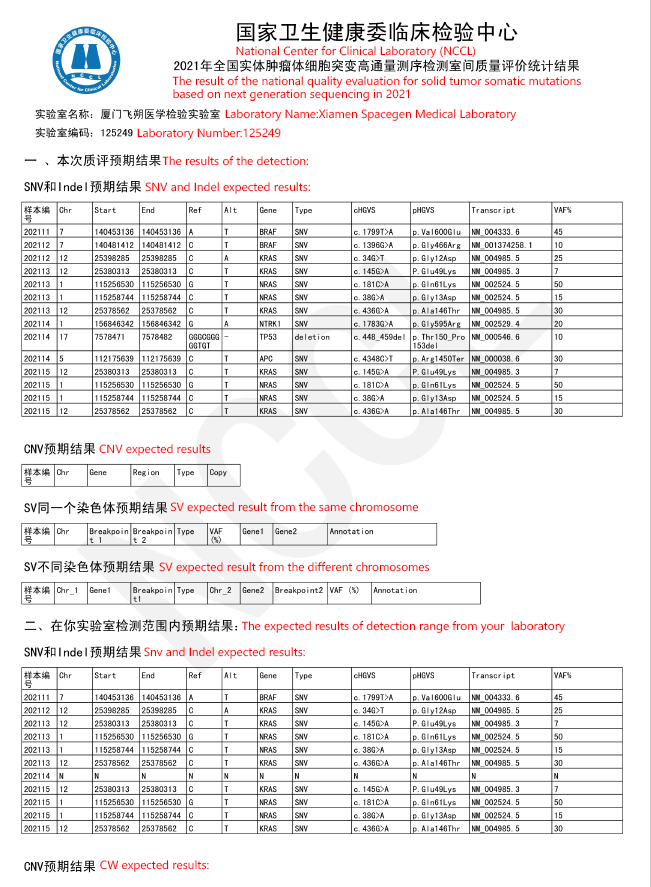
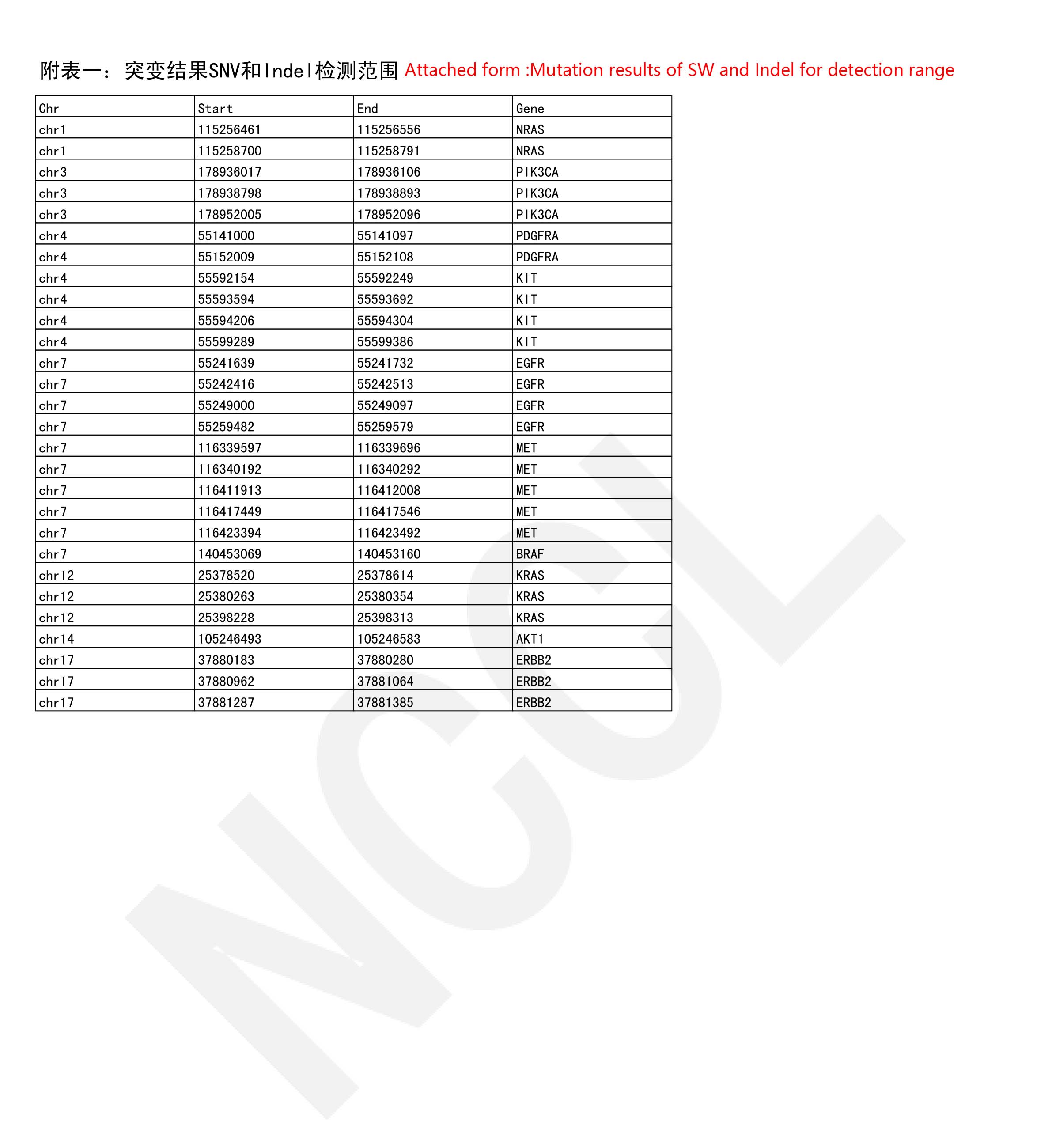
It is reported that the national quality evaluationfor solid tumor somatic mutations Program is an important means to ensure thequality of detection in clinical laboratories. It is mainly designed todetermine the detection ability for the next generation sequencing of solidtumor somatic mutations in each participating laboratory, to discover theproblems (common /special) in the detection process in the laboratory, to promotethe improvement of the detection level for each laboratory.
The qualifications for the pre- study activity of theexternal quality evaluation are strict, and the laboratories that signed up shouldfollow the below conditions: 1) Should have the ability to carry out the nationalquality evaluation for solid tumor somatic mutations independently, and do notaccept registrations from external laboratories. (2) Gene detection quantity ≥100,detection area ≥0.5Mb,do notaccept registration from laboratories that only detect hotspot mutations. 3)Participated in the NCCL " the external quality evaluation for nationalsolid tumor somatic mutation based on next generation sequencing" projectand qualified.
Spacegen Medical Laboratory
In the future, Spacegen Medical Laboratory willcontinue to focus on the field of gene technology services, implementhigh-standard, high-demand experimental standard procedures, provide stable andreliable gene sequencing services, and strive to become a high-quality nationalgene technology company.