Current location: Home > NEWS > Industry news
NEWS
PRODUCTS
Clinical study of the new generation of ROS1 inhibitor Taletrectinib Abstract
News source: Release time:[2021-10-25]
Onthe theme report of the CSCO Annual Conference on the morning of September 25,the Professor Zhou Caicun from Shanghai Pulmonary Hospital Reported thepreliminary results of the Phase II study (TRUST study, NCT04395677) ofTaletrectinib (DS-6051b/AB-106) in the treatment of ROS1-fusion-positive NSCLCpatients. This is not the first time Taletrectinib has appeared in the publiceye. At this year's ASCO annual meeting, AnHeart Pharmaceutical and Innoventjointly published the preliminary results of the TRUST study, which caused ahuge sensation.
ROS1Fusion
ROS1 gene (ROSProto-Oncogene 1, Receptor Tyrosine Kinase) is located on chromosome 6 ,encoding a receptor tyrosine kinase . ROS1 isoften involved in the gene rearrangement of a variety of human cancers. Theencoded fusion protein contains the continuously activated ROS1 kinase domainand drives cell proliferation. In NSCLC, about 1~2% of patients have ROS1rearrangement. ROS1 rearrangement occurs mostly in relatively young,non-smokers with lung cancer.
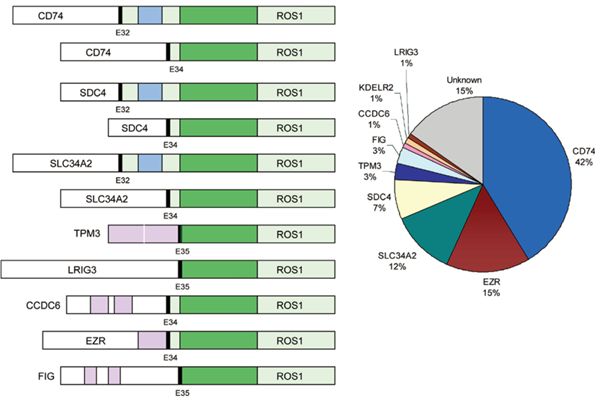
Thereare many fusion partners in ROS1, of which CD74 is the most common(42%)
ROS1Treatment of Fusion Patients
According to the recommendations of theNCCN guidelines, the first-line treatment options for NSCLC patients withpositive ROS1 rearrangement include crizotinib, entrectinib, and ceritinib, thedrugs for later-line treatment include lauratinib and entrectinib.
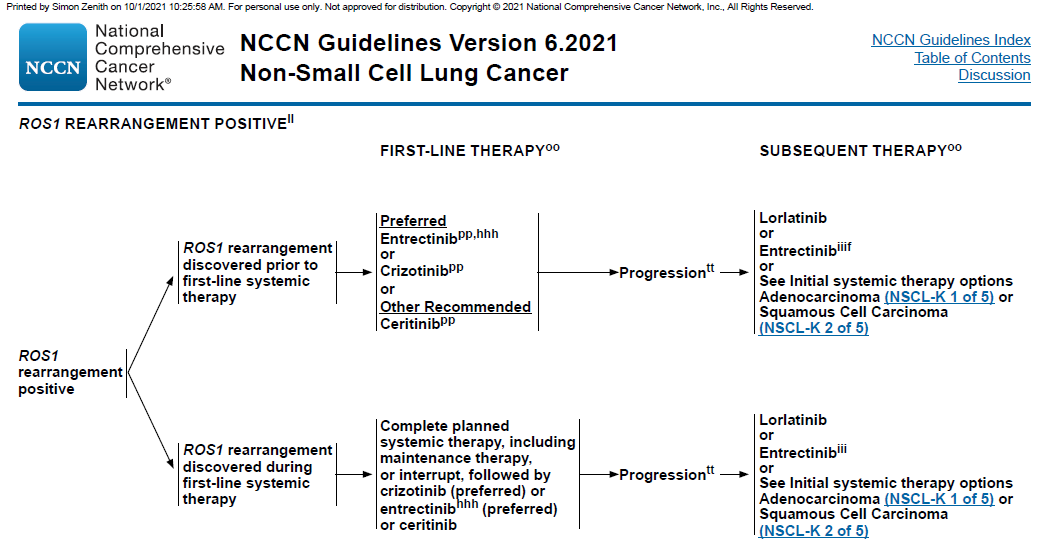
NCCN NSCLC Guidelines Version 6.2021
But in China, patients do not have somany choices. According to the CSCO guidelines, crizotinib is recommended asthe first-line treatment for patients with advanced NSCLC with ROS1 fusion,crizotinib is recommended for grade I, entrectinib is recommended for gradeIII. However, entretinib has not been approved for marketing in China. Inaddition, there are no targeted drugs available, only chemotherapy or theoption of participating in clinical research.
Ros1 fusion positivenon-small cell lung cancer
Stage | Stratification | GradeⅠRecommendation | GradeⅡRecommendation | GradeⅢRecommendation |
First-line treatment of stage Ⅵ ROS1 fusion NSCLCa,b,c | crizotinib (classification 3) [1] | Platinum-containing dual-drug chemotherapy±bevacizumab(non-squamous cell carcinoma) [2]d(classification 2A) | entrectinib(classification 3) [3] | |
Phase Ⅵ ROS1 fusion NSCLC second-line treatment | small progression or CNS progression | original TKI treatment + local treatment [4,5] (classification 2A) | participate in ROS1 inhibitor clinical research [7-11] (classification 3) | |
extensive progress | Platinum-containing dual-drug chemotherapy±bevacizumab(non-squamous cell carcinoma) [2,6](classification 2A) | |||
Phase Ⅵ ROS1 fusion NSCLC third-line treatment | PS=0~2 | single drug chemotherapy (classification 2A) | single drug chemotherapy + bevacizumab(non-squamous cell carcinoma) [12] (classification 2A) Participate in ROS1 inhibitor clinical research [7-11] (classification 3) |
CSCO NSCLC Diagnosis and Treatment Guide 2021 Edition
DrugResistance of Crizotinib
Although crizotinib is effective in ROS1 fusion-positiveNSCLC patients, most patients will acquire resistance. The main reason forcrizotinib resistance in ROS1-positive patients is the occurrence of resistancemutations in ROS1, among which the common ones are G2302R (41%), D2033N (6%)and S1986F (6%).
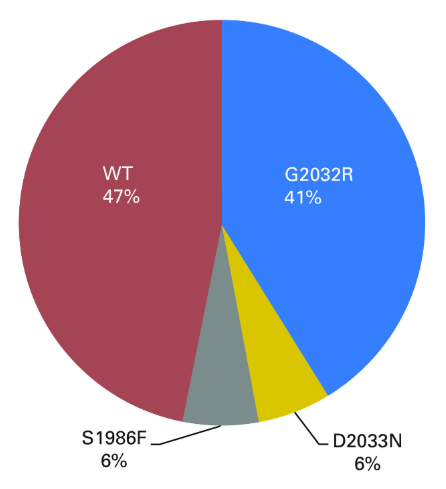
ROS1 resistance mutations and frequency
ThePreclinical studies of Taletrectinib
Taletrectinib (DS-6051b, domestic codeAB-106) is an NTRK/ROS1 inhibitor, originally developed by Japan's DaiichiSankyo Co., Ltd., AnHeart has obtained global development and commercializationrights, exclusively authorizing Innovent Joint development andcommercialization in China.
In preclinical studies, it was found thatDS-6051b can inhibit ROS1 and NTRK kinases at nanomolar concentrations, andexhibit anti-tumor activity in a human tumor cell line (PDC) model of ROS1rearrangement. The Ba/F3-CD74-ROS1 mutant model was used to test the activityof DS-6051b in vitro. DS-6051b effectively inhibited the growth of L1951R,L2026M, S1986F and G2032R mutant Ba/F3 cells. Especially for G2302R cells withthe highest mutation frequency, only DS-6051 can inhibit its growth at a lowerconcentration.
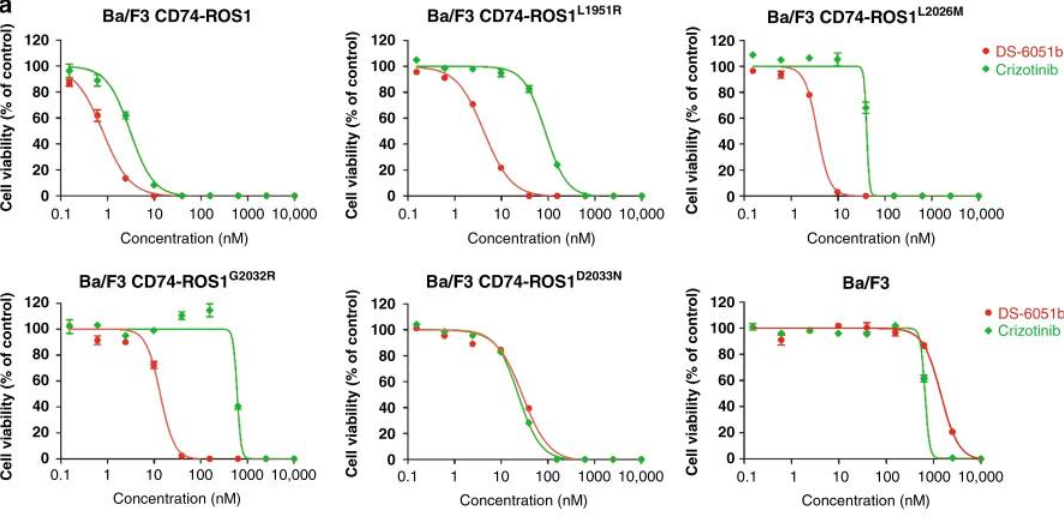
Sensitivity of CD74-ROS1 rearrangement(wild-type or crizotinib-resistant mutant) Ba/F3 cell line to DS-6051b and crizotinib
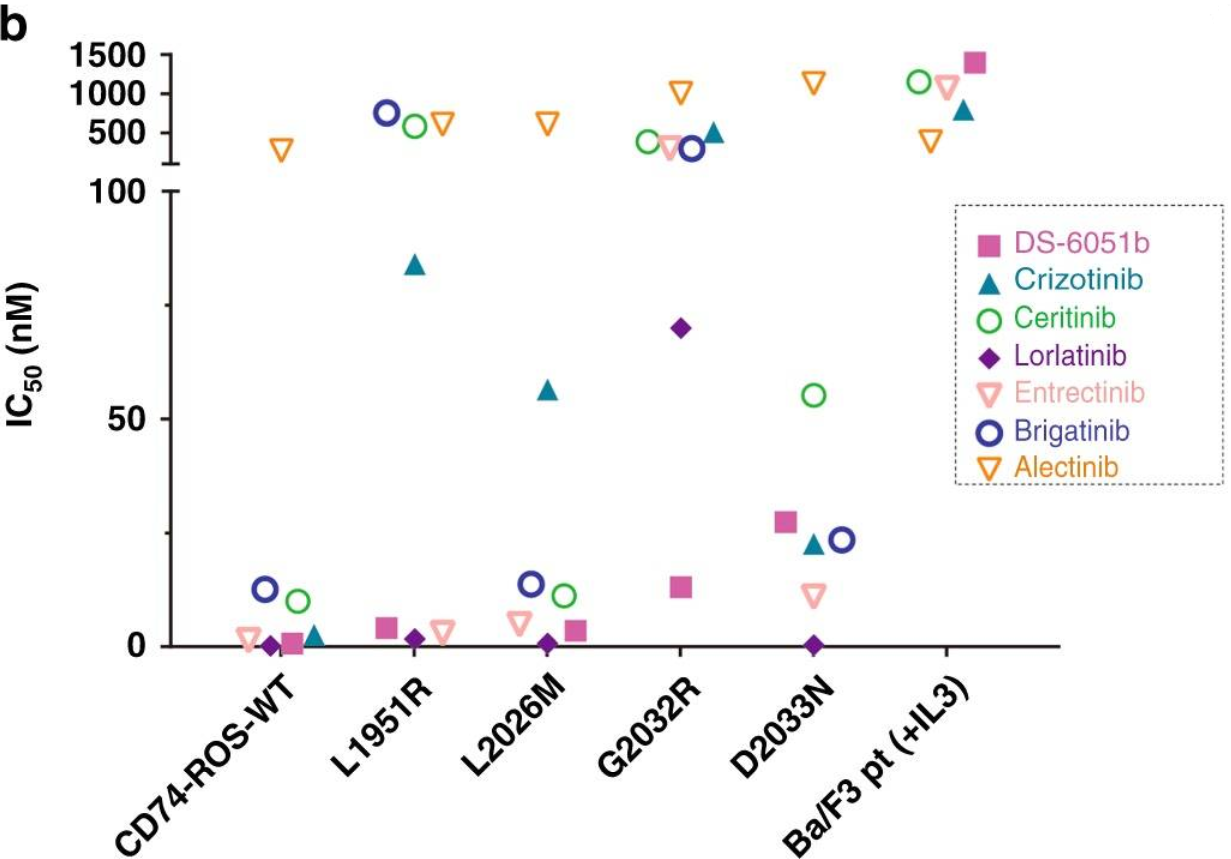
Sensitivity of the above Ba/F3 cell linesto various ROS1 inhibitors and ALK inhibitors
Animal experiments have also confirmedthat DS-6051b has a significant inhibitory effect on ROS1 wild-type and themain drug-resistant mutation G2032R; while the mutant type that uses loratinibor entrectinib, the tumor is still growing.
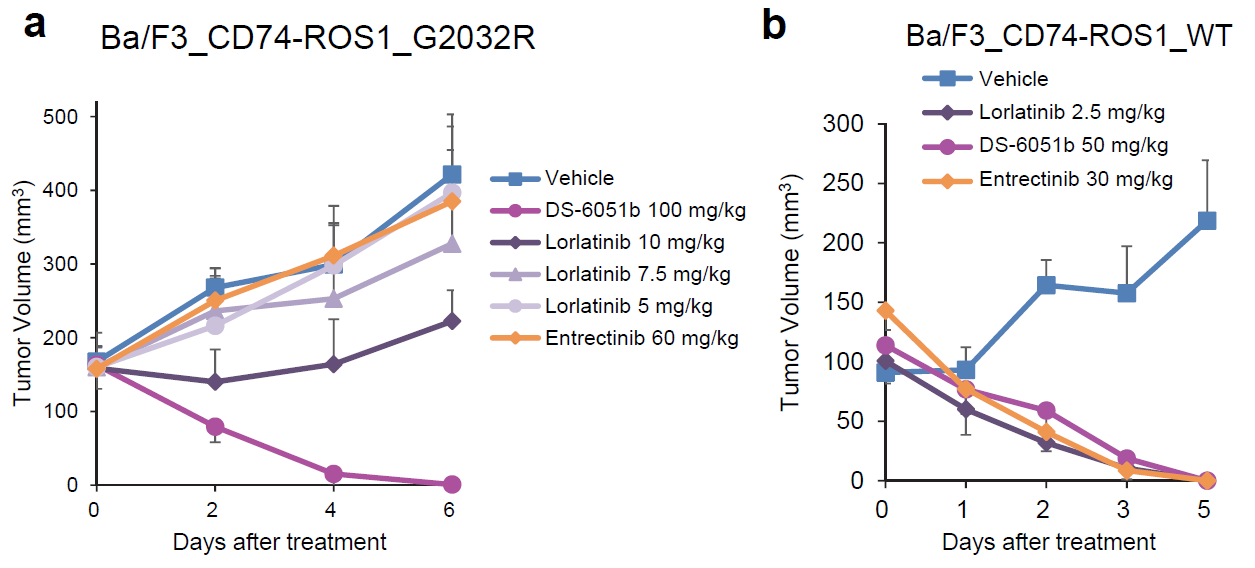
DS-6051b inhibits the growth of G2032Rmutant tumors in animal models
Clinical Study of Taletrectinib
AnHeart has successively carried outphase 1 clinical studies in the United States (NCT02279433) and Japan(NCT02675491) to evaluate the safety and tolerability of DS-6051b, and finallydetermined the clinical recommended dose of 600 mg QD. Immediately afterwards,AnHeart has successively carried out phase 2 clinical studies in China(NCT04395677) and globally (NCT04919811). This year’s CSCO conference reportedthe preliminary results of a phase 2, multi-center, single-arm, open-labelstudy of AB-106 in the treatment of advanced NSCLC patients with ROS1fusion-positive in China. The study consists of 2 parts. Part 1 (N=6) evaluatesthe safety and pharmacokinetic characteristics of AB-106 by using two doses of400 mg QD and 600 mg QD to confirm that 600 mg QD is the best dose.Part 2(N=100) evaluates the efficacy and safety of AB-106 by using 600 mg QD. As ofJune 16, 2021, a total of 69 subjects have been enrolled.
Preliminary Research Results:
Among 21patients who have not received crizotinib treatment, the confirmed ORR was90.5% (19/21) [ORR was 74% when crizotinib was approved (NCT01154140)], and theDCR was 90.5% ( 19/21), PFS is not yet mature.
Among the16 patients who had received crizotinib treatment, the confirmed ORR was 43.8%(7/16), and the DCR was 75.0% (12/16). Three of the patients were confirmed tobe positive for the ROS1 G2032R resistance mutation. All three patients showedtumor shrinkage. Two of the patients achieved PR and one patient was in SD.
Patientswith positive ROS1 fusion are prone to have brain metastases, but it isdifficult for crizotinib to pass through the blood-brain barrier. Amongpatients with evaluable brain metastases before enrollment, the investigator'sestimated intracranial objective response rate (iORR) was 83.3% (5/6).
AB-106 iswell tolerated, and treatment-related AE events mainly include gastrointestinaladverse events and reversible AST and ALT elevations.
According to the NCCN guidelines, NGS detection of DNA level maymiss ROS1 fusion. Spacegen's Human Oncology Multi-Gene Mutations Detection Kit(Guo Xie Zhu Zhun 20203400094) adopts the patented technology RingCap® forlibrary construction to detect ROS1 andother gene fusions at the RNA level; the operation is simple, the report timeis short, it is the best companion diagnostic product for the first and secondgeneration ROS1 inhibitors.

NCCN NSCLC Guidelines Version 6.2021
Reference
[1] Novel targets in non-small cell lungcancer: ROS1 and RET fusions [J]. Oncologist.2013;18(7):865-75.
[2] NCCN NSCLC Guidelines Version 5.2021
[3] CSCO NSCLC Diagnosis and TreatmentGuidelines 2021 Edition
[4] Patterns of Metastatic Spread andMechanisms of Resistance to Crizotinib in ROS1-Positive Non-Small-Cell LungCancer [J]. JCO Precis Oncol. 2017;2017:PO.17.00063.
[5] The new-generation selectiveROS1/NTRK inhibitor DS-6051b overcomes crizotinib resistant ROS1-G2032Rmutation in preclinical models [J]. Nat Commun. 2019 Aug 9;10(1):3604.
[6] Taletrectinib(AB-106): Anew generation of potent ROS1/NTRK inhibitors in the treatment of ROS1-positivenon-small cell lung cancer Phase II study (TRUST study) report